- 1Department of Biochemistry, Cell and Molecular Biology, West African Centre for Cell Biology of Infectious Pathogens (WACCBIP), University of Ghana, Accra, Ghana
- 2Department of Medical Microbiology, University of Ghana Medical School, College of Health Sciences, University of Ghana, Accra, Ghana
- 3Department of Virology, Noguchi Memorial Institute for Medical Research, University of Ghana, Accra, Ghana
- 4Department of Medical Laboratory Sciences, School of Biomedical and Allied Health Sciences, University of Ghana, Accra, Ghana
- 5Department of Paediatrics, Yale School of Medicine, Yale University, New Haven, CT, United States
- 6Department of Population, Family and Reproductive Health, School of Public Health, University of Ghana, Accra, Ghana
Abstract
People living with HIV (PLWH) usually suffer from co-infections and co-morbidities including respiratory tract infections. SARS-CoV-2 has been reported to cause respiratory infections. There are uncertainties in the disease severity and immunological response among PLWH who are co-infected with COVID-19. This review outlines the current knowledge on the clinical outcomes and immunological response to SARS-CoV-2 among PLWH. Literature was searched in Google scholar, Scopus, PubMed, and Science Direct conforming with the Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) guidelines from studies published from January 2020 to June 2023. A total of 81 studies from 25 countries were identified, and RT-PCR was used in confirming COVID-19 in 80 of the studies. Fifty-seven studies assessed risk factors and clinical outcomes in HIV patients co-infected with COVID-19. Thirty-nine of the studies indicated the following factors being associated with severe outcomes in HIV/SARS-CoV-2: older age, the male sex, African American race, smoking, obesity, cardiovascular diseases, low CD4+ count, high viral load, tuberculosis, high levels of inflammatory markers, chronic kidney disease, hypertension, diabetes, interruption, and delayed initiation of ART. The severe outcomes are patients’ hospitalization, admission at intensive care unit, mechanical ventilation, and death. Twenty (20) studies, however, reported no difference in clinical presentation among co-infected compared to mono-infected individuals. Immune response to SARS-CoV-2 infection was investigated in 25 studies, with some of the studies reporting high levels of inflammatory markers, T cell exhaustion and lower positive conversion rate of IgG in PLWH. There is scanty information on the cytokines that predisposes to severity among HIV/SARS-CoV-2 co-infected individuals on combined ART. More research work should be carried out to validate co-infection-related cytokines and/or immune markers to SARS-CoV-2 among PLWH.
Impact statement
People living with HIV often experience co-infections and co-morbidities, including respiratory tract infections. SARS-CoV-2 which is known to cause severe respiratory tract infections, has been reported among PLWH. There are, however, conflicting reports on HIV patients co-infected with SARS-CoV-2 with scanty information on other human coronaviruses. Studies that reported on clinical outcomes and immunological responses were reviewed through search engines and PRISMA selection criteria, with most studies indicating similar risk factors that predisposes to disease severity. High levels of inflammatory markers, T cell exhaustion and lower positive conversion rate of IgG were identified in individuals co-infected with HIV/SARS-CoV-2. Research on cytokines and immune markers in HIV/SARS-CoV-2 co-infected individuals on combined ART is limited and therefore, necessitating further validation.
Introduction
People living with HIV (PLWH) usually suffer from co-infections and co-morbidities including respiratory tract infections, renal impairment, hypertension, diabetes, obesity, hyperlipidemia, chronic viral hepatitis, and non-AIDS-defining malignancies among others [1, 2]. These co-infections and co-morbidities tend to limit the efficacy of the antiretroviral therapy [3]. Respiratory tract infections are of a major concern due to the compromised immune state of PLWH that makes them vulnerable to severe diseases [4].
In 2019, the novel SARS-CoV-2, a new coronavirus broke out in China, also known as (COVID-19). As of September 2023, WHO reported 770,875,433 confirmed cases of COVID-19, and 6,959,316 deaths [5] spreading throughout the globe. SARS-CoV-2 has been reported to also cause more severe RTIs in HIV patients [6, 7]. Co-infections in humans have become a topic among researchers with wide interest to know their clinical importance [8, 9].
PLWH infected with COVID-19, are thought to have more complicated clinical presentations due to immunodeficiency and immune imbalance [6]. Research has reported COVID-19 in PLWH to be severe [10]. Other studies however, indicated similarity in prevalence and deleterious outcomes among both the co-infected and mono-infected [11, 12]. Bhaskaran and others reported an increased COVID-19 mortality and morbidity risk among PLWH [13], but other researchers were not convinced about this assertion and cautioned its authenticity [14].
T cell immune activation and some cytokines play a role in HIV progression [15]. COVID-19 infection has also been investigated to be associated with some immune profiles [16]. This usually leads to a cytokine storm where cytokines are then released to control inflammation causing more white blood cells to accumulate, creating a cycle of inflammation thereby damaging the lung cells. This indicates that co-infections of these HIV/SARS-CoV-2 conditions among humans may lead to harmful immunological response and a poor prognosis of disease.
This review paper sought to outline the current knowledge of clinical outcomes and immunological response to SARS-CoV-2 infection among PLWH. It also sought to identify gaps in relation to this coinfection study.
Materials and methods
Selection criteria
All studies reporting on clinical outcomes and immune response among PLWH co-infected with SARS-CoV-2 were included. All immunological studies with observational studies, cohort studies, case reports, randomized controlled trials, and case series were also included. All studies meeting the above stated criteria, published from January 2020 to June 2023 and in the English language were included. Studies that do not address clinical outcomes and immunological response among PLWH co-infected with COVID-19 were excluded from this review. Letters to editors, editorials, commentaries, and brief reports that did not report on any clinical data were excluded. Literature reviews, systematic review and meta-analysis data were excluded.
Data sources and search strategy
We searched in PubMed, Google scholar, Scopus, and Science Direct using relevant terms such as “SARS-CoV-2” or “COVID-19” and ‘HIV or “Human Immunodeficiency Syndrome” or AIDS or “Acquired Immunodeficiency Disease” or PLWH or “People Living With HIV.” We then applied extra filters to access articles on “Immunological Response” or “Immune Characterization” or “Immunological Profiles” or “T-Cell Activation and Cytokines Profiles” and “Outcomes.” Also, eligible studies were identified by scanning references by manual search.
Study selection
Titles were imported into Endnotes for every search, and duplicates were eliminated. Titles and abstracts were used by two researchers to independently check records for eligibility. The complete texts of any publications that were thought to be possibly eligible were then retrieved, evaluated, and unanimously chosen to be included in the study. Conflicts were arbitrated by a third investigator or resolved by consensus.
Data extraction and synthesis
Extracted data were imputed into a table. All data were in English language. Studies were curated by sampling date, study design, study place, study participants, assay type, additional tests, author, and year of publication.
Results
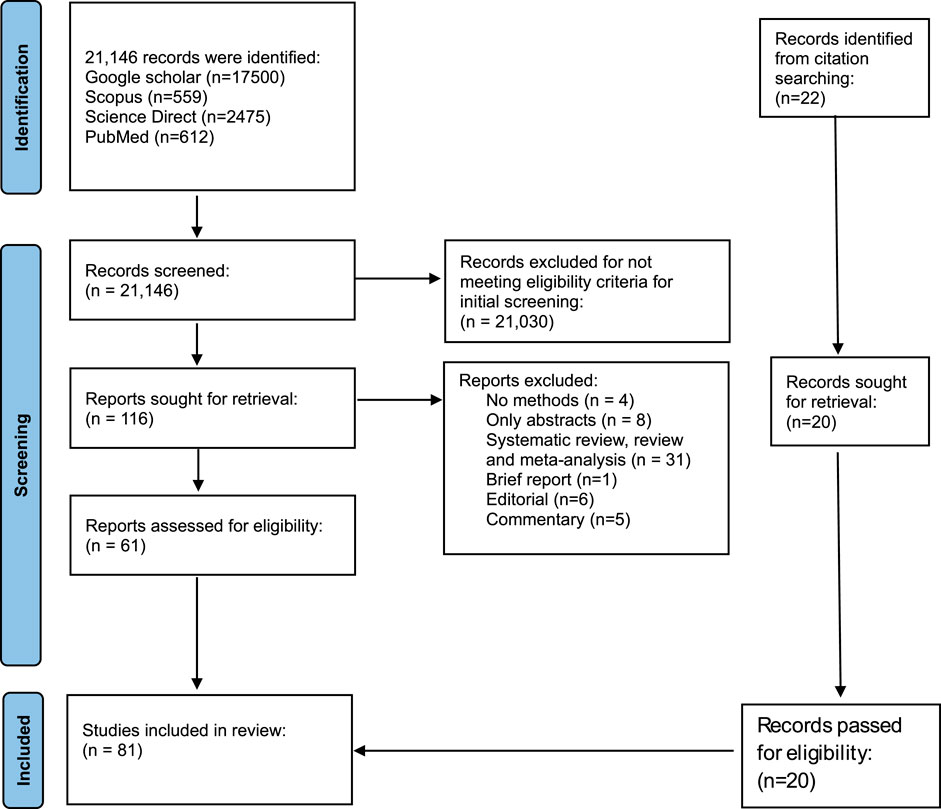
Studies selection was done using PRISMA guidelines (Figure 1). Databases searches (Google scholar: 17,500, Scopus: 559, ScienceDirect: 2,475, PubMed: 612) identified a total of 21,146. Eighty-three (81) studies met the eligibility criteria after the selection process (Figure 1). Endnote Software was used to remove duplicates. Also, studies that did not meet the eligibility criteria in the initial screening were 21,031. Fifty-five (55) studies were excluded due to the following reasons: No methods (4), only abstract (8), systematic review, literature review, meta-analysis (3), Brief report (1), editorial (6) and commentary (5). A manual scanning of references resulted in 22 additional reports. Eighty-one studies were included in this analysis.
Characteristics of included studies
Studies were identified in 25 countries in this review with United State of America contributing 17 of the included studies. Other countries where studies were carried out included South Africa (12), Italy (10), China (9), Spain (7), United Kingdom (5), France (3), Russia (2) and Brazil (2). Only one study was identified in the following countries (Korea, UAE, Iran, Germany, Japan, Guinea Bissau, Netherlands, Taiwan, Sweden, Israel, Belgium, Zambia, and Indonesia). Study participants included HIV patients, HIV naïve groups, COVID-19 patients. All studies were conducted among HIV patients. RT-PCR was used in confirming human coronaviruses in 99% of the studies. ELISA techniques were also used in 14 of the studies, followed by flow cytometry (n = 9), neutralization assay (n = 5), ELISpot (n = 4).
Fifty-seven studies have assessed clinical outcomes in HIV patients that were co-infected with SARS-CoV-2 (Table 1). Immune response to COVID-19 infection was investigated by 25 studies (Table 2). 18 studies were made up of brief reports, case reports and editorials with clinical and laboratory data. All the studies were carried out from 2020 to 2023.
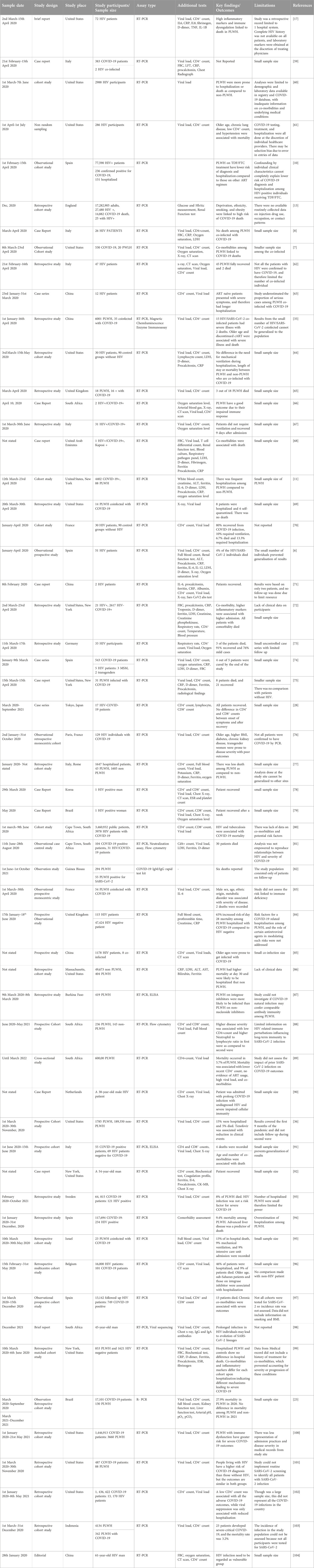
Table 1. Summary of clinical outcomes on HIV and SARS-CoV-2 co-infection studies and their spatial distribution.
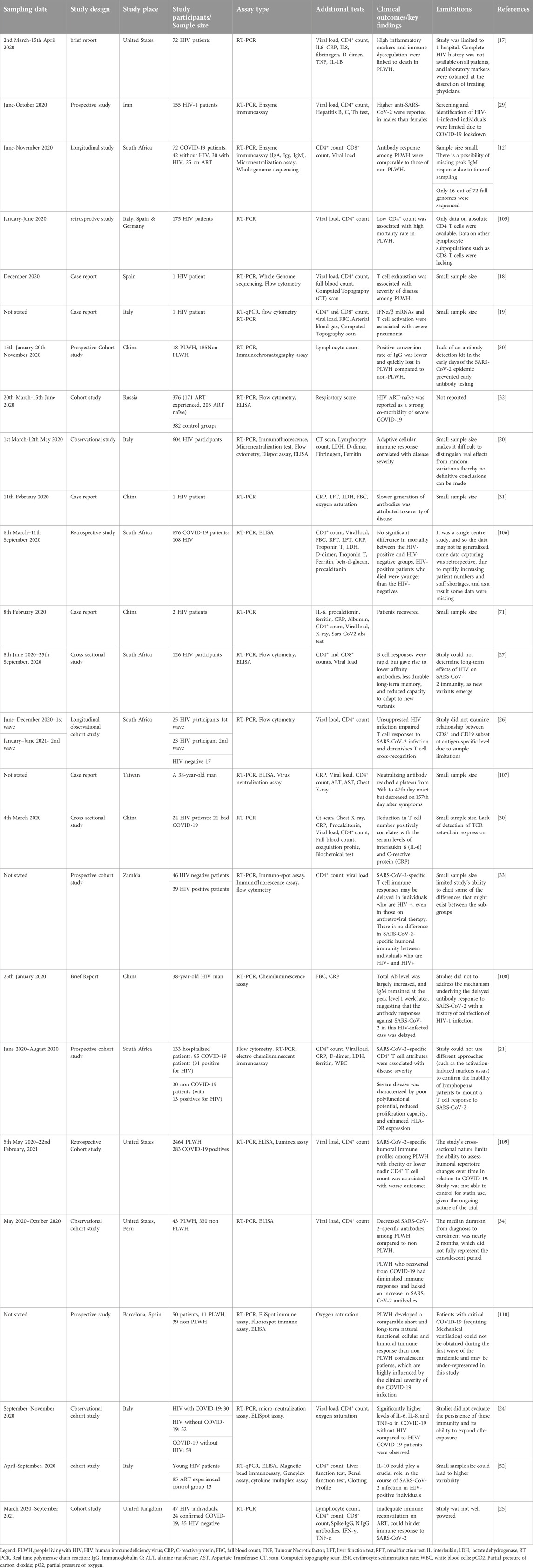
Table 2. Summary of immunological response on HIV and SARS-CoV-2 co-infection studies and their spatial distribution.
Thirty (38) studies reported the following risk factors as associated with severity of diseases (Table 1). This includes older age, higher BMI, male sex, deprivation, ethnicity, obesity, smoking, Tuberculosis, chronic kidney disease, higher inflammatory markers, diabetes, cardiovascular disease, lung cancer, African American, high viral load, low CD4+ count, high neutrophil-lymphocyte ratio, discontinued ART usage and some ART regimen. Twenty studies however indicated that clinical presentations among the co-infected were the same as the general population therefore there was low risk of disease severity (Table 1).
Twenty-five studies looked at immunological responses (Table 2), out of which four suggested that high inflammatory markers and immune dysregulation are linked to severity of disease and death among people who are coinfected with HIV/SARS-CoV-2 and are on ART, even though the ART is supposed to help with HIV viral suppression and immune reconstitution [17–22]. HIV/SARS-CoV-2 individuals with higher pro-inflammatory markers such as C-reactive protein (CRP), IL-8, IL-6 presented with disease severity and higher mortality than those who recovered [17]. Three other studies on co-infections linked reduction of T cell numbers to increased IL-6, IL-8, and CRP levels, causing a cytokine storm [23–25]. Among the co-infected individuals, unsuppressed HIV hampers T cell cross-recognition and responses to SARS-CoV-2 infection, and thereby leading to severe outcomes [26, 27]. The pre-symptom and post recovery CD4+, and CD8+ counts showed no significant difference between PLWH and HIV negative individuals who are infected with SARS-CoV-2 [28]. PLWH saw a brief decline in CD4+, and CD8+ counts during the acute phase of COVID-19 with the CD4+/CD8+ ratio remaining unchanged [11, 28].
Most of the studies were either retrospective or prospective with one time point sample collection, therefore, no subsequent CD4+ counts and viral loads to determine relationship with clinical outcomes. Two of the studies were longitudinal with one study investigating two waves of SARS-CoV-2 infection [26] and the other following up for a period of 3 months on HIV/SARS-CoV-2 patients [12]. Snyman et al., indicated in their study that anti-SARS-CoV-2 IgM, IgG, and IgA levels in non-HIV individuals and PLWH on full HIV suppression on ART have similar seroconversion rates [12]. The conversion rate of anti-SARS-CoV-2 IgG was lower and quickly lost in PLWH as compared to HIV negative persons who are SARS-CoV-2 positive [29–31]. Three of the studies indicated that slower generation of anti SARS CoV2 antibodies were attributed to increased COVID-19 severity among PLWH [32–34].
Discussion
We conducted a scoping review to assess specific COVID-19 clinical outcomes and immune response in patients with human immunodeficiency virus (PLWH) and identify gaps. Hospitalisation risk, intensive care unit admission, mechanical ventilation and mortality were the four categories identified as clinical outcomes. Our review showed varied reports on risk of hospitalisation, ICU admission, mechanical ventilation and mortality in cohort studies, case series, and case reports. PLWH who died exhibited higher levels of soluble immune activation and inflammation markers, which are linked to disease severity in COVID-19 [22]. Individuals with non-suppressed HIV viremia have reported lower levels of antibodies against SARS-CoV-2 in their humoral response [35]. Some studies however, associated low risk of hospitalization and death to Tenofovir usage as compared to those on other regimen [35–37].
Immune response to SARS-CoV-2 infection among PLWH on ART
ART does not eradicate HIV completely but significantly reduces morbidity and mortality associated with the virus [38]. ART may also reduce the severity of COVID-19 through immunological reconstitution, although these effects have not yet been confirmed [10, 36, 39]. PLWH with mild COVID-19 presentation, in the presence of high proinflammatory markers, suggested that certain antiretroviral drugs were protective against severity of COVID-19 disease [20]. A study in Russia among 376 HIV/COVID-19 patients (171 without ART and 205 with ART) suggested that elevated anti-inflammatory markers such as IL-10 and TGFβ, reduced CD4+/CD8+ cell ratios led to an increase in exhausted T cells in ART naïve patients. This led to Adverse Respiratory Distress Syndrome among the ART naïve group [32]. Sharov also reiterated that in the presence of uninterrupted ART, HIV patients do not progress to severe SARS-CoV-2 infection [32]. Other studies hypothesized that specific ART (NRTIs, NNRTIs and PI) predisposes to severe COVID-19 but no conclusive findings have been made because of studies involving smaller sample size and inconsistent cases and reports [40, 41].
Signaling pathway of HIV/SARS-CoV-2 coinfection
Viral infections interact mainly with the activated Signal Transducer and Activators of Transcription 1, 2, and 3 (STAT1, STAT2 and STAT3) to release pro-inflammatory cytokines to eliminate viruses [42]. The IL-6-JAK-STAT3 axis is significantly linked to the onset of severe COVID-19 [43, 44]. The dimerized epidermal growth factor receptor (EGFR) can tyrosine-phosphorylate STAT3, which is elevated in cases of acute lung injury [45] and in cases where STAT1 is lacking [46, 47]. As a result, in COVID-19, EGFR signalling may develop into a different pathway that stimulates STAT3 when lung damage occurs, and SARS-CoV-2 infection significantly reduces IFN-I production [48]. This aberrant transcriptional rewiring towards STAT3 may lead to the symptoms most typically reported in hospitalised COVID-19 patients: fast coagulopathy/thrombosis, proinflammatory conditions, profibrotic state, and T cell lymphopenia [49].
Some HIV proteins have been reported to inhibit effective IFNα signalling by degrading certain components of the JAK/STAT signalling pathway like STAT1 and STAT3 [50]. The impaired JAK/STAT signalling pathway is however restored in the presence of uninterrupted combined Antiretroviral therapy (cART) for more than 6 months [51]. Per our search, we found one study available on HIV/COVID-19 signalling pathway that investigated STAT3 but did not look at other STAT pathways [52], and therefore creates a gap that needs to be researched. Understanding the viral co-infection, immune response, and signalling pathway dynamics will help identify particulate markers that predisposes to severity of disease.
Oxidative stress responses among HIV/SARS-CoV-2 coinfection
Hyperactivation of STAT3 affect various biological and physiological functions, leading to oxidative stress (OxS) and poor prognosis of disease [22]. Oxidative stress (OxS) comes about by accumulating reactive oxygen and nitrogen species, which are free radicals that causes injury to organs. Under physiological conditions, these OxS are wiped out by antioxidants especially glutathione (GSH) [53]. Glutathione are endogenous intracellular antioxidants that neutralizes free radical released due to oxidative stress [54]. Deficiency in GSH however, leads to high levels of OxS due to compromised antioxidant defences [55]. Oxidative stress has been studied in HIV or SARS-CoV 2 alone with higher levels reported in each disease [55–58]. There is however scanty information on oxidative stress among HIV/COVID-19 patients, hence the need to investigate if the presence of ART usage affects oxidative stress response.
Limitations
There is lack of information on cellular immunity in other hCoVs apart from COVID-19 co-infection. Cytokine have been studied extensively in HIV or COVID-19 alone but not as a co-infection. The oxidative stress levels among HIV/SARS-CoV-2 co-infection are yet to be studied although research has been done for other co-morbidities or co-infections.
Conclusion
This study highlights the paucity of clinical and immunological data on HIV/SARS-CoV-2 co-infection in sub-Saharan Africa, even though this region has the highest HIV prevalence. Review shows conflicting reports on severity of the co-infection. HIV/SARS-CoV-2 severity and outcomes appear to be worse, when coexisting age-related comorbidities and CD4 + T-cell depletion is present. Discontinued or no evidence of ART usage have also been shown to increase disease severity, which needs to be studied further to ascertain its authenticity.
CD4+ T cell lymphopenia in both diseases is influenced by various mechanisms including direct attacks, immune activation, and redistribution of CD4+ T cells. Cytokines investigation will help identify markers that are implicated in disease severity among HIV/SARS-CoV-2 patients. Further investigation is needed to confirm co-infection-associated cytokines and/or immunological markers to SARS-CoV-2 in PLWH.
Author contributions
Study conception was performed by EA, EB, ET, EP, KT, and OQ. Original draft preparation was performed by EA, PA, LA, and MA-P. Methodology was performed by EA, PA, LA, and MA-P. EB, ET, EP, KT, and OQ critically reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded in part by the Fogarty International Center of National Institute of Alcohol Abuse and Alcoholism of the National Institutes of Health (NIH) [D43 TW011526], the World Bank African Centres of Excellence grant [WACCBIP+NCDs: Awandare], and the Science for Africa Foundation to Developing Excellence in Leadership, Training and Science in Africa (DELTAS Africa) programme [DEL-22-014] with support from Wellcome and the UK Foreign, Commonwealth and Development Office (FCDO) which is part of the EDCPT2 programme supported by the European Union. The content of the research is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, World Bank, Wellcome Trust, or the FCDO.
Acknowledgments
The authors acknowledge the support of the West African Centre for Cell Biology of Infectious Pathogens (WACCBIP) and the HIVComRT.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Schouten, J, Wit, FW, Stolte, IG, Kootstra, NA, van der Valk, M, Geerlings, SE, et al. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis (2014) 59(12):1787–97. doi:10.1093/cid/ciu701
2. Önen, NF, Overton, ET, Seyfried, W, Stumm, ER, Snell, M, Mondy, K, et al. Aging and HIV infection: a comparison between older HIV-infected persons and the general population. HIV Clin trials (2010) 11(2):100–9. doi:10.1310/hct1102-100
3. Brown, TT, and Guaraldi, G. Multimorbidity and burden of disease. Interdiscip Top Gerontol Geriatr (2017) 42:59–73. doi:10.1159/000448544
4. Kanwugu, ON, and Adadi, P. HIV/SARS-CoV-2 coinfection: a global perspective. J Med Virol (2021) 93(2):726–32. doi:10.1002/jmv.26321
6. Vizcarra, P, Pérez-Elías, MJ, Quereda, C, Moreno, A, Vivancos, MJ, Dronda, F, et al. Description of COVID-19 in HIV-infected individuals: a single-centre, prospective cohort. The lancet HIV (2020) 7(8):e554–e564. doi:10.1016/s2352-3018(20)30164-8
7. Collins, LF, Moran, CA, Oliver, NT, Moanna, A, Lahiri, CD, Colasanti, JA, et al. Clinical characteristics, comorbidities and outcomes among persons with HIV hospitalized with coronavirus disease 2019 in Atlanta, Georgia. AIDS (London, England) (2020) 34(12):1789–94. doi:10.1097/qad.0000000000002632
8. Calza, L, Bon, I, Tadolini, M, Borderi, M, Colangeli, V, Badia, L, et al. COVID-19 in patients with HIV-1 infection: a single-centre experience in northern Italy. Infection (2021) 49(2):333–7. doi:10.1007/s15010-020-01492-7
9. Hartley, DM, and Perencevich, EN. Public health interventions for COVID-19: emerging evidence and implications for an evolving public health crisis. Jama (2020) 323(19):1908–9. doi:10.1001/jama.2020.5910
10. Del Amo, J, Polo, R, Moreno, S, Díaz, A, Martínez, E, Arribas, JR, et al. Antiretrovirals and risk of COVID-19 diagnosis and hospitalization in HIV-positive persons. Epidemiology (Cambridge, Mass.) (2020) 31(6):e49–e51. doi:10.1097/ede.0000000000001235
11. Sigel, K, Swartz, T, Golden, E, Paranjpe, I, Somani, S, Richter, F, et al. Coronavirus 2019 and people living with human immunodeficiency virus: outcomes for hospitalized patients in New York City. Clin Infect Dis (2020) 71(11):2933–8. doi:10.1093/cid/ciaa880
12. Snyman, J, Hwa, SH, Krause, R, Muema, D, Reddy, T, Ganga, Y, et al. Similar antibody responses against severe acute respiratory syndrome coronavirus 2 in individuals living without and with human immunodeficiency virus on antiretroviral therapy during the first South African infection wave. Clin Infect Dis (2022) 75(1):e249–e256. doi:10.1093/cid/ciab758
13. Bhaskaran, K, Rentsch, CT, MacKenna, B, Schultze, A, Mehrkar, A, Bates, CJ, et al. HIV infection and COVID-19 death: a population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. The lancet HIV (2021) 8(1):e24–e32. doi:10.1016/s2352-3018(20)30305-2
14. Waters, LJ, and Pozniak, AL. COVID-19 death in people with HIV: interpret cautiously. The Lancet HIV (2021) 8(1):e2–e3. doi:10.1016/s2352-3018(20)30332-5
15. Scagnolari, C, and Antonelli, G. Type I interferon and HIV: subtle balance between antiviral activity, immunopathogenesis and the microbiome. Cytokine Growth Factor Rev (2018) 40:19–31. doi:10.1016/j.cytogfr.2018.03.003
16. Zhou, Z, Ren, L, Zhang, L, Zhong, J, Xiao, Y, Jia, Z, et al. Heightened innate immune responses in the respiratory tract of COVID-19 patients. Cell host & microbe (2020) 27(6):883–90. doi:10.1016/j.chom.2020.04.017
17. Ho, H-e., Peluso, MJ, Margus, C, Matias Lopes, JP, He, C, Gaisa, MM, et al. Clinical outcomes and immunologic characteristics of coronavirus disease 2019 in people with human immunodeficiency virus. J Infect Dis (2021) 223(3):403–8. doi:10.1093/infdis/jiaa380
18. Álvarez, H, Ruiz-Mateos, E, Juiz-González, PM, Vitallé, J, Viéitez, I, Vázquez-Friol, MC, et al. SARS-CoV-2 evolution and spike-specific CD4+ T-cell response in persistent COVID-19 with severe HIV immune suppression. Microorganisms (2022) 10(1):143. doi:10.3390/microorganisms10010143
19. d’Ettorre, G, Recchia, G, Ridolfi, M, Siccardi, G, Pinacchio, C, Innocenti, GP, et al. Analysis of type I IFN response and T cell activation in severe COVID-19/HIV-1 coinfection: a case report. Medicine (2020) 99(36):e21803. doi:10.1097/md.0000000000021803
20. Mondi, A, Cimini, E, Colavita, F, Cicalini, S, Pinnetti, C, Matusali, G, et al. COVID-19 in people living with HIV: clinical implications of dynamics of the immune response to SARS-CoV-2. J Med Virol (2021) 93(3):1796–804. doi:10.1002/jmv.26556
21. Riou, C, du Bruyn, E, Stek, C, Daroowala, R, Goliath, RT, Abrahams, F, et al. Relationship of SARS-CoV-2-specific CD4 response to COVID-19 severity and impact of HIV-1 and tuberculosis coinfection. J Clin Invest (2021) 131(12):e149125. doi:10.1172/jci149125
22. Ruan, Q, Yang, K, Wang, W, Jiang, L, and Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med (2020) 46(5):846–8. doi:10.1007/s00134-020-05991-x
23. Sales, TLS, Souza-Silva, MVR, Delfino-Pereira, P, Neves, JVB, Sacioto, MF, Assis, VC, et al. COVID-19 outcomes in people living with HIV: peering through the waves. Clinics (2023) 78:100223. doi:10.1016/j.clinsp.2023.100223
24. Vergori, A, Boschini, A, Notari, S, Lorenzini, P, Castilletti, C, Colavita, F, et al. SARS-CoV-2 specific immune response and inflammatory profile in advanced HIV-infected persons during a COVID-19 outbreak. Viruses (2022) 14(7):1575. doi:10.3390/v14071575
25. Alrubayyi, A, Gea-Mallorquí, E, Touizer, E, Hameiri-Bowen, D, Kopycinski, J, Charlton, B, et al. Characterization of humoral and SARS-CoV-2 specific T cell responses in people living with HIV. Nat Commun (2021) 12(1):5839. doi:10.1038/s41467-021-26137-7
26. Nkosi, T, Ndhlovu, ZM, Chasara, C, Papadopoulos, AO, Nguni, TL, Karim, F, et al. Unsuppressed HIV infection impairs T cell responses to SARS-CoV-2 infection and abrogates T cell cross-recognition. Elife (2022) 11:e78374. doi:10.7554/elife.78374
27. Krause, R, Snyman, J, Shi-Hsia, H, Muema, D, Karim, F, Ganga, Y, et al. HIV skews the SARS-CoV-2 B cell response towards an extrafollicular maturation pathway. Elife (2022) 11:e79924. doi:10.7554/eLife.79924
28. Adachi, E, Saito, M, Nagai, H, Ikeuchi, K, Koga, M, Tsutsumi, T, et al. Transient depletion of T cells during COVID-19 and seasonal influenza in people living with HIV. J Med Virol (2022) 94(5):1789–91. doi:10.1002/jmv.27543
29. Garshasbi, S, Bokharaei-Salim, F, Khanaliha, K, Kiani, SJ, Kalantari, S, Jamshidi Makiani, M, et al. SARS-CoV-2 infection in Iranian people living with human immunodeficiency virus-1 infection. Jundishapur J Microbiol (2022) 15(1). doi:10.5812/jjm.121929
30. Liu, Y, Xiao, Y, Wu, S, Marley, G, Ming, F, Wang, X, et al. People living with HIV easily lose their immune response to SARS-CoV-2: result from a cohort of COVID-19 cases in Wuhan, China. BMC Infect Dis (2021) 21(1):1029–7. doi:10.1186/s12879-021-06723-2
31. Wang, M, Luo, L, Bu, H, and Xia, H. One case of coronavirus disease 2019 (COVID-19) in a patient co-infected by HIV with a low CD4+ T-cell count. Int J Infect Dis (2020) 96:148–50. doi:10.1016/j.ijid.2020.04.060
32. Sharov, KS. HIV/SARS-CoV-2 co-infection: T cell profile, cytokine dynamics and role of exhausted lymphocytes. Int J Infect Dis (2021) 102:163–9. doi:10.1016/j.ijid.2020.10.049
33. Ngalamika, O, Lidenge, SJ, Mukasine, MC, Kawimbe, M, Kamanzi, P, Ngowi, JR, et al. SARS-CoV-2-specific T cell and humoral immunity in individuals with and without HIV in an African population: a prospective cohort study. Int J Infect Dis (2023) 127:106–15. doi:10.1016/j.ijid.2022.12.009
34. Schuster, DJ, Karuna, S, Brackett, C, Wesley, M, Li, SS, Eisel, N, et al. Lower SARS-CoV-2–specific humoral immunity in people living with HIV-1 recovered from nonhospitalized COVID-19. JCI insight (2022) 7(21):e158402. doi:10.1172/jci.insight.158402
35. Huang, J, Xie, N, Hu, X, Yan, H, Ding, J, Liu, P, et al. Epidemiological, virological and serological features of coronavirus disease 2019 (COVID-19) cases in people living with human immunodeficiency virus in Wuhan: a population-based cohort study. Clin Infect Dis (2021) 73(7):e2086–e2094. doi:10.1093/cid/ciaa1186
36. Lea, AN, Leyden, WA, Sofrygin, O, Marafino, BJ, Skarbinski, J, Napravnik, S, et al. Human immunodeficiency virus status, Tenofovir exposure, and the risk of poor coronavirus disease 19 outcomes: real-world analysis from 6 United States cohorts before vaccine rollout. Clin Infect Dis (2023) 76(10):1727–34. doi:10.1093/cid/ciad084
37. Del Amo, J, Polo, R, Moreno, S, Jarrín, I, and Hernán, MA. SARS-CoV-2 infection and coronavirus disease 2019 severity in persons with HIV on antiretroviral treatment. Aids (2022) 36(2):161–8. doi:10.1097/qad.0000000000003132
38. Lohse, N, and Obel, N. Update of survival for persons with HIV infection in Denmark. Ann Intern Med (2016) 165(10):749–50. doi:10.7326/l16-0091
39. Suwanwongse, K, and Shabarek, N. Clinical features and outcome of HIV/SARS-CoV-2 coinfected patients in the Bronx, New York city. J Med Virol (2020) 92(11):2387–9. doi:10.1002/jmv.26077
40. Costenaro, P, Minotti, C, Barbieri, E, Giaquinto, C, and Donà, D. SARS-CoV-2 infection in people living with HIV: a systematic review. Rev Med Virol (2021) 31(1):1–12. doi:10.1002/rmv.2155
41. Mirzaei, H, McFarland, W, Karamouzian, M, and Sharifi, H. COVID-19 among people living with HIV: a systematic review. AIDS Behav (2021) 25:85–92. doi:10.1007/s10461-020-02983-2
42. Luo, Y, Alexander, M, Gadina, M, O’Shea, JJ, Meylan, F, and Schwartz, DM. JAK-STAT signaling in human disease: from genetic syndromes to clinical inhibition. J Allergy Clin Immunol (2021) 148(4):911–25. doi:10.1016/j.jaci.2021.08.004
43. Giamarellos-Bourboulis, EJ, Netea, MG, Rovina, N, Akinosoglou, K, Antoniadou, A, Antonakos, N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell host & microbe (2020) 27(6):992–1000. doi:10.1016/j.chom.2020.04.009
44. Hirano, T, and Murakami, M. COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity (2020) 52(5):731–3. doi:10.1016/j.immuni.2020.04.003
45. Finigan, JH, Downey, GP, and Kern, JA. Human epidermal growth factor receptor signaling in acute lung injury. Am J Respir Cel Mol Biol (2012) 47(4):395–404. doi:10.1165/rcmb.2012-0100tr
46. Venkataraman, T, Coleman, CM, and Frieman, MB. Overactive epidermal growth factor receptor signaling leads to increased fibrosis after severe acute respiratory syndrome coronavirus infection. J Virol (2017) 91(12):e00182-17. doi:10.1128/jvi.00182-17
47. Yang, L, Xu, J, Guo, L, Guo, T, Zhang, L, Feng, L, et al. Porcine epidemic diarrhea virus-induced epidermal growth factor receptor activation impairs the antiviral activity of type I interferon. J Virol (2018) 92(8):e02095-17. doi:10.1128/jvi.02095-17
48. Hadjadj, J, Yatim, N, Barnabei, L, Corneau, A, Boussier, J, Smith, N, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science (2020) 369(6504):718–24. doi:10.1126/science.abc6027
49. Matsuyama, T, Kubli, SP, Yoshinaga, SK, Pfeffer, K, and Mak, TW. An aberrant STAT pathway is central to COVID-19. Cel Death Differ (2020) 27(12):3209–25. doi:10.1038/s41418-020-00633-7
50. Gargan, S, Ahmed, S, Mahony, R, Bannan, C, Napoletano, S, O'Farrelly, C, et al. HIV-1 promotes the degradation of components of the type 1 IFN JAK/STAT pathway and blocks anti-viral ISG induction. EBioMedicine (2018) 30:203–16. doi:10.1016/j.ebiom.2018.03.006
51. Liu, M-Q, Zhao, M, Kong, WH, Tang, L, Wang, F, Zhu, ZR, et al. Combination antiretroviral therapy (cART) restores HIV-1 infection-mediated impairment of JAK-STAT signaling pathway. Oncotarget (2017) 8(14):22524–33. doi:10.18632/oncotarget.15121
52. Vanetti, C, Trabattoni, D, Stracuzzi, M, Amendola, A, Fappani, C, Rubinacci, V, et al. Immunological characterization of HIV and SARS-CoV-2 coinfected young individuals. Cells (2021) 10(11):3187. doi:10.3390/cells10113187
53. Ballatori, N, Krance, SM, Notenboom, S, Shi, S, Tieu, K, and Hammond, CL. Glutathione dysregulation and the etiology and progression of human diseases. bchm (2009) 390:191–214. doi:10.1515/bc.2009.033
54. Yan, Y, Yang, Y, Wang, F, Ren, H, Zhang, S, Shi, X, et al. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res Care (2020) 8(1):e001343. doi:10.1136/bmjdrc-2020-001343
55. Kumar, P, Osahon, O, Vides, DB, Hanania, N, Minard, CG, and Sekhar, RV. Severe glutathione deficiency, oxidative stress and oxidant damage in adults hospitalized with COVID-19: implications for GlyNAC (glycine and N-acetylcysteine) supplementation. Antioxidants (2021) 11(1):50. doi:10.3390/antiox11010050
56. Pace, GW, and Leaf, CD. The role of oxidative stress in HIV disease. Free Radic Biol Med (1995) 19(4):523–8. doi:10.1016/0891-5849(95)00047-2
57. Repetto, M, Reides, C, Gomez Carretero, ML, Costa, M, Griemberg, G, and Llesuy, S. Oxidative stress in blood of HIV infected patients. Clinica Chim Acta (1996) 255(2):107–17. doi:10.1016/0009-8981(96)06394-2
58. Ntyonga-Pono, M-P. COVID-19 infection and oxidative stress: an under-explored approach for prevention and treatment? Pan Afr Med J (2020) 35(2):12. doi:10.11604/pamj.2020.35.2.22877
59. Sasset, L, Di Meco, E, Cavinato, S, and Cattelan, AM. Coinfection of severe acute respiratory syndrome coronavirus 2 and HIV in a teaching hospital: still much to learn. AIDS (2020) 34(11):1694–6. doi:10.1097/qad.0000000000002609
60. Tesoriero, JM, Swain, CAE, Pierce, JL, Zamboni, L, Wu, M, Holtgrave, DR, et al. COVID-19 outcomes among persons living with or without diagnosed HIV infection in New York State. JAMA Netw open (2021) 4(2):e2037069. doi:10.1001/jamanetworkopen.2020.37069
61. Dandachi, D, Geiger, G, Montgomery, MW, Karmen-Tuohy, S, Golzy, M, Antar, AAR, et al. Characteristics, comorbidities, and outcomes in a multicenter registry of patients with human immunodeficiency virus and coronavirus disease 2019. Clin Infect Dis (2021) 73(7):e1964–e1972. doi:10.1093/cid/ciaa1339
62. Gervasoni, C, Meraviglia, P, Riva, A, Giacomelli, A, Oreni, L, Minisci, D, et al. Clinical features and outcomes of patients with human immunodeficiency virus with COVID-19. Clin Infect Dis (2020) 71(16):2276–8. doi:10.1093/cid/ciaa579
63. Hu, Y, Ma, J, Huang, H, and Vermund, SH. Coinfection with HIV and SARS-CoV-2 in Wuhan, China: a 12-person case series. J acquired immune deficiency syndromes (2020) 85(1):1–5. doi:10.1097/QAI.0000000000002424
64. Stoeckle, K, Johnston, CD, Jannat-Khah, DP, Williams, SC, Ellman, TM, Vogler, MA, et al. COVID-19 in hospitalized adults with HIV. Open Forum Infect Dis (2020) 7:ofaa327. Oxford University Press US. doi:10.1093/ofid/ofaa327
65. Madge, S, Barber, TJ, Hunter, A, Bhagani, S, Lipman, M, and Burns, F. Descriptive account of 18 adults with known HIV infection hospitalised with SARS-CoV-2 infection. Sex Transm Infections (2021) 97(5):392–3. doi:10.1136/sextrans-2020-054660
66. Mitha, M, Maharaj, A, and Nyamande, K. People living with HIV and COVID-19: a report on 2 clinical cases from South Africa. Afr J Thorac Crit Care Med (2020) 26(2):59–60. doi:10.7196/ajtccm.2020.v26i2.078
67. Calza, L, Bon, I, Borderi, M, Colangeli, V, Borioni, A, Re, MC, et al. COVID-19 outcomes in patients with uncontrolled HIV-1 infection. JAIDS J Acquired Immune Deficiency Syndromes (2021) 86(1):e15–e17. doi:10.1097/qai.0000000000002537
68. Qasim, A, Mansour, M, Kousa, O, Awad, D, Abuhazeem, B, Millner, P, et al. A case of coronavirus disease 2019 in acquired immunodeficiency syndrome patient: a case report and review of the literature. Intractable Rare Dis Res (2020) 9(4):256–9. doi:10.5582/irdr.2020.03081
69. Gudipati, S, Brar, I, Murray, S, McKinnon, JE, Yared, N, and Markowitz, N. Descriptive analysis of patients living with HIV affected by COVID-19. JAIDS J Acquired Immune Deficiency Syndromes (2020) 85(2):123–6. doi:10.1097/qai.0000000000002450
70. Isernia, V, Julia, Z, Le Gac, S, Bachelard, A, Landman, R, Lariven, S, et al. SARS-COV2 infection in 30 HIV-infected patients followed-up in a French University Hospital. Int J Infect Dis (2020) 101:49–51. doi:10.1016/j.ijid.2020.09.1436
71. Zhang, J-C, Yu, XH, Ding, XH, Ma, HY, Cai, XQ, Kang, SC, et al. New HIV diagnoses in patients with COVID-19: two case reports and a brief literature review. BMC Infect Dis (2020) 20(1):771–10. doi:10.1186/s12879-020-05480-y
72. Karmen-Tuohy, S, Carlucci, PM, Zervou, FN, Zacharioudakis, IM, Rebick, G, Klein, E, et al. Outcomes among HIV-positive patients hospitalized with COVID-19. JAIDS J Acquired Immune Deficiency Syndromes (2020) 85:6–10. doi:10.1097/qai.0000000000002423
73. Härter, G, Spinner, CD, Roider, J, Bickel, M, Krznaric, I, Grunwald, S, et al. COVID-19 in people living with human immunodeficiency virus: a case series of 33 patients. Infection (2020) 48:681–6. doi:10.1007/s15010-020-01438-z
74. Blanco, JL, Ambrosioni, J, Garcia, F, Martínez, E, Soriano, A, Mallolas, J, et al. COVID-19 in patients with HIV: clinical case series. The lancet HIV (2020) 7(5):e314–e316. doi:10.1016/s2352-3018(20)30111-9
75. Shalev, N, Scherer, M, LaSota, ED, Antoniou, P, Yin, MT, Zucker, J, et al. Clinical characteristics and outcomes in people living with human immunodeficiency virus hospitalized for coronavirus disease 2019. Clin Infect Dis (2020) 71(16):2294–7. doi:10.1093/cid/ciaa635
76. Bachelard, A, Sautereau, A, Digumber, M, Isernia, V, Phung, B, Lehur, AC, et al. Risk factors associated with severe/critical COVID-19 in people living with HIV-1. Int J Infect Dis (2022) 122:152–4. doi:10.1016/j.ijid.2022.05.055
77. Gagliardini, R, Vergori, A, Lorenzini, P, Cicalini, S, Pinnetti, C, Mazzotta, V, et al. Characteristics and outcomes of COVID-19-related hospitalization among PLWH. J Clin Med (2022) 11(6):1546. doi:10.3390/jcm11061546
78. Kim, J-Y, Kim, JM, and Peck, KR. The first case of an HIV patient diagnosed with COVID-19 in Korea. J Korean Med Sci (2020) 35(39):e358. doi:10.3346/jkms.2020.35.e358
79. Cipolat, MM, and Sprinz, E. COVID-19 pneumonia in an HIV-positive woman on antiretroviral therapy and undetectable viral load in Porto Alegre, Brazil. Braz J Infect Dis (2020) 24:455–7. doi:10.1016/j.bjid.2020.07.009
80. Davies, M-A. HIV and risk of COVID-19 death: a population cohort study from the Western Cape Province, South Africa. MedRxiv (2020). Available from: https://doi.org/10.1101/2020.07.02.20145185.
81. Du Bruyn, E, Stek, C, Daroowala, R, Said-Hartley, Q, Hsiao, M, Schafer, G, et al. Effects of tuberculosis and/or HIV-1 infection on COVID-19 presentation and immune response in Africa. Nat Commun (2023) 14(1):188. doi:10.1038/s41467-022-35689-1
82. Dutschke, A, Wejse, C, Nanque, J, Medina, C, Hønge, B, Jespersen, S, et al. SARS-CoV-2 seroprevalence among people living with HIV in Guinea–Bissau. Public Health (2022) 209:36–8. doi:10.1016/j.puhe.2022.05.017
83. Etienne, N, Karmochkine, M, Slama, L, Pavie, J, Batisse, D, Usubillaga, R, et al. HIV infection and COVID-19: risk factors for severe disease. AIDS (London, England) (2020) 34(12):1771–4. doi:10.1097/qad.0000000000002651
84. Geretti, AM, Stockdale, AJ, Kelly, SH, Cevik, M, Collins, S, Waters, L, et al. Outcomes of COVID-19 related hospitalisation among people with HIV in the ISARIC WHO Clinical Characterisation Protocol UK Protocol: prospective observational study. MedRxiv (2020). Available from: https://doi.org/10.1101/2020.08.07.20170449.
85. Guo, W, Ming, F, Dong, Y, Zhang, Q, Zhang, X, Mo, P, et al. A survey for COVID-19 among HIV/AIDS patients in two districts of Wuhan, China (2020). Available from: http://dx.doi.org/10.2139/ssrn.3550029.
86. Hadi, YB, Naqvi, SF, Kupec, JT, and Sarwari, AR. Characteristics and outcomes of COVID-19 in patients with HIV: a multicentre research network study. AIDS (London, England) (2020) 34(13):F3–F8. doi:10.1097/qad.0000000000002666
87. Kaboré, OD, Poda, A, Ouattara, CA, Michodigni, FN, Belem, AA, Sawadogo, Y, et al. Seroprevalence of SARS-CoV-2 IgG and associated factors among people living with HIV over the first 12 months following the outbreak of COVID-19 in Burkina Faso, a sub-Saharan African country. Plos one (2023) 18(6):e0286665. doi:10.1371/journal.pone.0286665
88. Karim, F, Gazy, I, Cele, S, Zungu, Y, Krause, R, Bernstein, M, et al. HIV status alters disease severity and immune cell responses in Beta variant SARS-CoV-2 infection wave. Elife (2021) 10:e67397. doi:10.7554/eLife.67397
89. Kassanjee, R, Davies, M, Ngwenya, O, Osei-Yeboah, R, Jacobs, T, Morden, E, et al. COVID-19 among adults living with HIV: correlates of mortality among public sector healthcare users in Western Cape, South Africa. J Int AIDS Soc (2023) 26(6):e26104. doi:10.1002/jia2.26104
90. Ketels, T, Gisolf, J, Claassen, M, Swanink, C, van Lochem, E, Moonen, L, et al. Short communication: prolonged COVID-19 infection in a patient with newly diagnosed HIV/AIDS. AIDS Res Hum retroviruses (2022) 38(5):399–400. doi:10.1089/aid.2021.0145
91. Maggiolo, F, Zoboli, F, Arosio, M, Valenti, D, Guarneri, D, Sangiorgio, L, et al. SARS-CoV-2 infection in persons living with HIV: a single center prospective cohort. J Med Virol (2021) 93(2):1145–9. doi:10.1002/jmv.26352
92. Mahmood, K, Rashed, ER, Oliveros, E, Chau, VQ, Hermle, T, Jacobs, S, et al. Predisposition or protection? COVID-19 in a patient on LVAD support with HIV/AIDS. JACC: Case Rep (2020) 2(9):1337–41. doi:10.1016/j.jaccas.2020.05.015
93. Möller, IK, Gisslén, M, Wagner, P, Sparén, P, and Carlander, C. COVID-19 hospitalization outcomes in adults by HIV status; a nation-wide register-based study. HIV Med (2023) 24:1045–55. doi:10.1111/hiv.13515
94. Moreno-Torres, V, de Mendoza, C, Martínez-Urbistondo, M, Mills, P, Treviño, A, de la Fuente, S, et al. Predictors of in-hospital mortality in HIV-infected patients with COVID-19. QJM: Int J Med (2023) 116(1):57–62. doi:10.1093/qjmed/hcac215
95. Nagarakanti, SR, Okoh, AK, Grinberg, S, and Bishburg, E. Clinical outcomes of patients with COVID-19 and HIV coinfection. J Med Virol (2021) 93(3):1687–93. doi:10.1002/jmv.26533
96. Nasreddine, R, Florence, E, Moutschen, M, Yombi, J, Goffard, J, Derdelinckx, I, et al. Clinical characteristics and outcomes of COVID-19 in people living with HIV in Belgium: a multicenter, retrospective cohort. J Med Virol (2021) 93(5):2971–8. doi:10.1002/jmv.26828
97. Nomah, DK, Reyes-Urueña, J, Díaz, Y, Moreno, S, Aceiton, J, Bruguera, A, et al. Sociodemographic, clinical, and immunological factors associated with SARS-CoV-2 diagnosis and severe COVID-19 outcomes in people living with HIV: a retrospective cohort study. The Lancet HIV (2021) 8(11):e701–e710. doi:10.1016/s2352-3018(21)00240-x
98. Peters, JL, Fall, A, Langerman, SD, El Asmar, M, Nakazawa, M, Mustapha, A, et al. Prolonged severe acute respiratory syndrome coronavirus 2 Delta variant shedding in a patient with AIDS: case report and review of the literature. Open Forum Infect Dis (2022) 9:ofac479. Oxford University Press US. doi:10.1093/ofid/ofac479
99. Rosenthal, EM, Rosenberg, ES, Patterson, W, Ferguson, WP, Gonzalez, C, DeHovitz, J, et al. Factors associated with SARS-CoV-2-related hospital outcomes among and between persons living with and without diagnosed HIV infection in New York State. PLoS One (2022) 17(5):e0268978. doi:10.1371/journal.pone.0268978
100. Sun, J, Patel, RC, Zheng, Q, Madhira, V, Olex, AL, Islam, JY, et al. COVID-19 disease severity among people with HIV infection or solid organ transplant in the United States: a nationally-representative, multicenter, observational cohort study. Medrxiv (2021). Avaialble from: https://doi.org/10.1101/2021.07.26.21261028.
101. Tang, ME, Gaufin, T, Anson, R, Zhu, W, Mathews, W, and Cachay, ER. People with HIV have a higher risk of COVID-19 diagnosis but similar outcomes to the general population. HIV Med (2022) 23(10):1069–77. doi:10.1111/hiv.13312
102. Yang, Y, and Iwasaki, A. Impact of chronic HIV infection on SARS-CoV-2 infection, COVID-19 disease and vaccines. Curr HIV/AIDS Rep (2022) 19:5–16. doi:10.1007/s11904-021-00590-x
103. Yunihastuti, E, Karjadi, TH, Widhani, A, Mahdi, HIS, Sundari, S, Hapsari, AF, et al. Incidence and severity prediction score of COVID-19 in people living with HIV (SCOVHIV): experience from the first and second waves of the pandemic in Indonesia. AIDS Res Ther (2022) 19(1):47–8. doi:10.1186/s12981-022-00472-1
104. Zhu, F, Cao, Y, Xu, S, and Zhou, M. Co-infection of SARS-CoV-2 and HIV in a patient in Wuhan city, China. J Med Virol (2020) 92:529–30. doi:10.1002/jmv.25732
105. Hoffmann, C, Casado, JL, Härter, G, Vizcarra, P, Moreno, A, Cattaneo, D, et al. Immune deficiency is a risk factor for severe COVID-19 in people living with HIV. HIV Med (2021) 22(5):372–8. doi:10.1111/hiv.13037
106. Venturas, J, Zamparini, J, Shaddock, E, Stacey, S, Murray, L, Richards, GA, et al. Comparison of outcomes in HIV-positive and HIV-negative patients with COVID-19. J Infect (2021) 83(2):217–27. doi:10.1016/j.jinf.2021.05.020
107. Liu, W-D, Hung, CC, Wang, JT, Tsai, MJ, Kuo, PH, Chao, TL, et al. Evolution of SARS-CoV-2 neutralizing antibody in an HIV-positive patient with COVID-19. J Formos Med Assoc (2021) 120(12):2186–90. doi:10.1016/j.jfma.2021.04.010
108. Zhao, J, Liao, X, Wang, H, Wei, L, Xing, M, Liu, L, et al. Early virus clearance and delayed antibody response in a case of coronavirus disease 2019 (COVID-19) with a history of coinfection with human immunodeficiency virus type 1 and hepatitis C virus. Clin Infect Dis (2020) 71(16):2233–5. doi:10.1093/cid/ciaa408
109. Schnittman, SR, Jung, W, Fitch, KV, Zanni, MV, McCallum, S, Lee, JSL, et al. Effect of host factors and COVID-19 infection on the humoral immune repertoire in treated HIV. JCI insight (2023) 8(5):e166848. doi:10.1172/jci.insight.166848
Keywords: people living with HIV, immunological response, clinical outcomes, COVID-19, HIV/SARS-CoV-2 coinfection
Citation: Amegashie EA, Asamoah P, Ativi LEA, Adusei-Poku M, Bonney EY, Tagoe EA, Paintsil E, Torpey K and Quaye O (2024) Clinical outcomes and immunological response to SARS-CoV-2 infection among people living with HIV. Exp. Biol. Med. 249:10059. doi: 10.3389/ebm.2024.10059
Received: 23 November 2023; Accepted: 22 February 2024;
Published: 02 April 2024.
Copyright © 2024 Amegashie, Asamoah, Ativi, Adusei-Poku, Bonney, Tagoe, Paintsil, Torpey and Quaye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Osbourne Quaye, oquaye@ug.edu.gh
 Esimebia Adjovi Amegashie1
Esimebia Adjovi Amegashie1 Osbourne Quaye
Osbourne Quaye