Abstract
People living with HIV (PLWH) usually suffer from co-infections and co-morbidities including respiratory tract infections. SARS-CoV-2 has been reported to cause respiratory infections. There are uncertainties in the disease severity and immunological response among PLWH who are co-infected with COVID-19. This review outlines the current knowledge on the clinical outcomes and immunological response to SARS-CoV-2 among PLWH. Literature was searched in Google scholar, Scopus, PubMed, and Science Direct conforming with the Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) guidelines from studies published from January 2020 to June 2023. A total of 81 studies from 25 countries were identified, and RT-PCR was used in confirming COVID-19 in 80 of the studies. Fifty-seven studies assessed risk factors and clinical outcomes in HIV patients co-infected with COVID-19. Thirty-nine of the studies indicated the following factors being associated with severe outcomes in HIV/SARS-CoV-2: older age, the male sex, African American race, smoking, obesity, cardiovascular diseases, low CD4+ count, high viral load, tuberculosis, high levels of inflammatory markers, chronic kidney disease, hypertension, diabetes, interruption, and delayed initiation of ART. The severe outcomes are patients’ hospitalization, admission at intensive care unit, mechanical ventilation, and death. Twenty (20) studies, however, reported no difference in clinical presentation among co-infected compared to mono-infected individuals. Immune response to SARS-CoV-2 infection was investigated in 25 studies, with some of the studies reporting high levels of inflammatory markers, T cell exhaustion and lower positive conversion rate of IgG in PLWH. There is scanty information on the cytokines that predisposes to severity among HIV/SARS-CoV-2 co-infected individuals on combined ART. More research work should be carried out to validate co-infection-related cytokines and/or immune markers to SARS-CoV-2 among PLWH.
Impact statement
People living with HIV often experience co-infections and co-morbidities, including respiratory tract infections. SARS-CoV-2 which is known to cause severe respiratory tract infections, has been reported among PLWH. There are, however, conflicting reports on HIV patients co-infected with SARS-CoV-2 with scanty information on other human coronaviruses. Studies that reported on clinical outcomes and immunological responses were reviewed through search engines and PRISMA selection criteria, with most studies indicating similar risk factors that predisposes to disease severity. High levels of inflammatory markers, T cell exhaustion and lower positive conversion rate of IgG were identified in individuals co-infected with HIV/SARS-CoV-2. Research on cytokines and immune markers in HIV/SARS-CoV-2 co-infected individuals on combined ART is limited and therefore, necessitating further validation.
Introduction
People living with HIV (PLWH) usually suffer from co-infections and co-morbidities including respiratory tract infections, renal impairment, hypertension, diabetes, obesity, hyperlipidemia, chronic viral hepatitis, and non-AIDS-defining malignancies among others [1, 2]. These co-infections and co-morbidities tend to limit the efficacy of the antiretroviral therapy [3]. Respiratory tract infections are of a major concern due to the compromised immune state of PLWH that makes them vulnerable to severe diseases [4].
In 2019, the novel SARS-CoV-2, a new coronavirus broke out in China, also known as (COVID-19). As of September 2023, WHO reported 770,875,433 confirmed cases of COVID-19, and 6,959,316 deaths [5] spreading throughout the globe. SARS-CoV-2 has been reported to also cause more severe RTIs in HIV patients [6, 7]. Co-infections in humans have become a topic among researchers with wide interest to know their clinical importance [8, 9].
PLWH infected with COVID-19, are thought to have more complicated clinical presentations due to immunodeficiency and immune imbalance [6]. Research has reported COVID-19 in PLWH to be severe [10]. Other studies however, indicated similarity in prevalence and deleterious outcomes among both the co-infected and mono-infected [11, 12]. Bhaskaran and others reported an increased COVID-19 mortality and morbidity risk among PLWH [13], but other researchers were not convinced about this assertion and cautioned its authenticity [14].
T cell immune activation and some cytokines play a role in HIV progression [15]. COVID-19 infection has also been investigated to be associated with some immune profiles [16]. This usually leads to a cytokine storm where cytokines are then released to control inflammation causing more white blood cells to accumulate, creating a cycle of inflammation thereby damaging the lung cells. This indicates that co-infections of these HIV/SARS-CoV-2 conditions among humans may lead to harmful immunological response and a poor prognosis of disease.
This review paper sought to outline the current knowledge of clinical outcomes and immunological response to SARS-CoV-2 infection among PLWH. It also sought to identify gaps in relation to this coinfection study.
Materials and methods
Selection criteria
All studies reporting on clinical outcomes and immune response among PLWH co-infected with SARS-CoV-2 were included. All immunological studies with observational studies, cohort studies, case reports, randomized controlled trials, and case series were also included. All studies meeting the above stated criteria, published from January 2020 to June 2023 and in the English language were included. Studies that do not address clinical outcomes and immunological response among PLWH co-infected with COVID-19 were excluded from this review. Letters to editors, editorials, commentaries, and brief reports that did not report on any clinical data were excluded. Literature reviews, systematic review and meta-analysis data were excluded.
Data sources and search strategy
We searched in PubMed, Google scholar, Scopus, and Science Direct using relevant terms such as “SARS-CoV-2” or “COVID-19” and ‘HIV or “Human Immunodeficiency Syndrome” or AIDS or “Acquired Immunodeficiency Disease” or PLWH or “People Living With HIV.” We then applied extra filters to access articles on “Immunological Response” or “Immune Characterization” or “Immunological Profiles” or “T-Cell Activation and Cytokines Profiles” and “Outcomes.” Also, eligible studies were identified by scanning references by manual search.
Study selection
Titles were imported into Endnotes for every search, and duplicates were eliminated. Titles and abstracts were used by two researchers to independently check records for eligibility. The complete texts of any publications that were thought to be possibly eligible were then retrieved, evaluated, and unanimously chosen to be included in the study. Conflicts were arbitrated by a third investigator or resolved by consensus.
Data extraction and synthesis
Extracted data were imputed into a table. All data were in English language. Studies were curated by sampling date, study design, study place, study participants, assay type, additional tests, author, and year of publication.
Results
Studies selection was done using PRISMA guidelines (Figure 1). Databases searches (Google scholar: 17,500, Scopus: 559, ScienceDirect: 2,475, PubMed: 612) identified a total of 21,146. Eighty-three (81) studies met the eligibility criteria after the selection process (Figure 1). Endnote Software was used to remove duplicates. Also, studies that did not meet the eligibility criteria in the initial screening were 21,031. Fifty-five (55) studies were excluded due to the following reasons: No methods (4), only abstract (8), systematic review, literature review, meta-analysis (3), Brief report (1), editorial (6) and commentary (5). A manual scanning of references resulted in 22 additional reports. Eighty-one studies were included in this analysis.
FIGURE 1
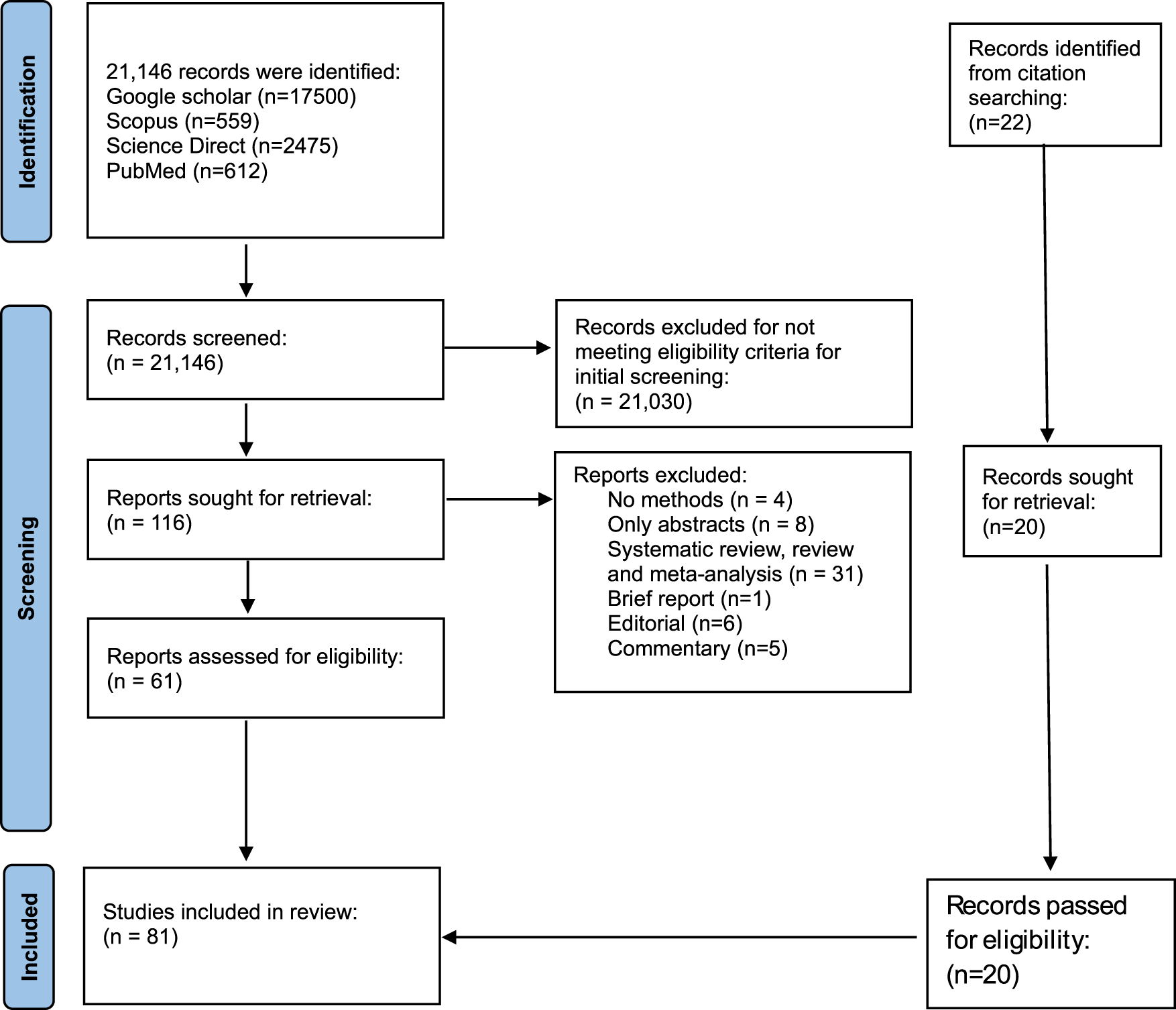
PRISMA flow diagram showing the selection criteria of studies.
Characteristics of included studies
Studies were identified in 25 countries in this review with United State of America contributing 17 of the included studies. Other countries where studies were carried out included South Africa (12), Italy (10), China (9), Spain (7), United Kingdom (5), France (3), Russia (2) and Brazil (2). Only one study was identified in the following countries (Korea, UAE, Iran, Germany, Japan, Guinea Bissau, Netherlands, Taiwan, Sweden, Israel, Belgium, Zambia, and Indonesia). Study participants included HIV patients, HIV naïve groups, COVID-19 patients. All studies were conducted among HIV patients. RT-PCR was used in confirming human coronaviruses in 99% of the studies. ELISA techniques were also used in 14 of the studies, followed by flow cytometry (n = 9), neutralization assay (n = 5), ELISpot (n = 4).
Fifty-seven studies have assessed clinical outcomes in HIV patients that were co-infected with SARS-CoV-2 (Table 1). Immune response to COVID-19 infection was investigated by 25 studies (Table 2). 18 studies were made up of brief reports, case reports and editorials with clinical and laboratory data. All the studies were carried out from 2020 to 2023.
TABLE 1
| Sample date | Study design | Study place | Study participants/Sample size | Assay type | Additional tests | Key findings/Outcomes | Limitations | References |
|---|---|---|---|---|---|---|---|---|
| 2nd March-15th April 2020 | brief report | United States | 72 HIV patients | RT-PCR | Viral load, CD4+ count, IL6, CRP, IL8, fibrinogen, D-dimer, TNF, IL-1B | High inflammatory markers and immune dysregulation linked to death in PLWH. | Study was a retrospective record limited to 1 hospital system. Complete HIV history was not available on all patients, and laboratory markers were obtained at the discretion of treating physicians | [17] |
| 21st February-15th April 2020 | Case report | Italy | 383 COVID-19 patients | RT-PCR | Viral load, CD4+ count, FBC, LFT, CRP, procalcitonin, Chest Radiograph | Not Reported | Small sample size | [59] |
| 2 HIV co-infected | ||||||||
| 1st March-7th June 2020 | cohort study | United States | 2988 HIV participants | RT-PCR | Viral load | PLWH were more prone to hospitalization or death as compared to non-PLWH. | Analyses were limited to demographic and laboratory data available in registry and COVID-19 database, with inadequate information on co-morbidities and underlying medical conditions | [60] |
| 1st April-1st July 2020 | Non random sampling | United States | 286 HIV participants | RT-PCR | Viral load, CD4+ count | Older age, chronic lung disease, low CD4+ count, and hypertension were associated with mortality | COVID-19 testing, treatment, and hospitalization were all done at the discretion of individual healthcare providers. There may be selection bias due to error in entries of data | [61] |
| 1st February-15th April 2020 | Observational cohort study | Spain | 77,590 HIV+ patients | RT-PCR | PLWH on TDF/FTC treatment have lower risk of diagnosis and hospitalization compared to those on other ART regimen | Confounding by individual clinical characteristics cannot completely explain lower risk of COVID-19 diagnosis and hospitalization among HIV-positive individuals receiving TDF/FTC. | [10] | |
| 236 confirmed positive for COVID-19, 151 hospitalized | ||||||||
| Dec, 2020 | Retrospective cohort study | England | 17,282,905 adults, 27,480 HIV +, 14,882 COVID-19 death, 25 with HIV+ | RT-PCR | Glucose and HbAic measurement, Renal Function test | Deprivation, ethnicity, smoking, and obesity were linked to high risk of COVID-19 death | There were no available routinely collected data on injection drug use, occupation, or contact patterns | [13] |
| March-April 2020 | Case Report | Italy | 26 HIV PATIENTS | RT-PCR | Viral load, CD4+count, FBC, CRP, Oxygen saturation, LDH | No death among PLWH co-infected with COVID-19 | Small sample size | [8] |
| 8th March-23rd April 2020 | Observational Cohort study | United States | 530 COVID-19, 20 PWLH | RT-PCR | Viral load, CD4+ count, Oxygen saturation, X-ray, CT scan | Co-morbidities among PLWH linked to COVID-19 deaths | Smaller sample size among the co-infected | [7] |
| 21st February-16th April 2020 | Retrospective study | Italy | 47 HIV patients | RT-PCR | x-ray, CT scan, Oxygen saturation, Viral load, CD4+ count | 45 PLWH fully recovered and 2 died | Not all the patients with HIV were confirmed to have COVID-19, and therefore limited the number of co-infected individual | [62] |
| 23rd January-31st March 2020 | Case series | China | 12 HIV patients | RT-PCR | CD4+ count, Viral load | ART naïve patients presented with severe symptoms, and therefore had longer hospitalization | Study underestimated the proportion of serious cases among PLWH co-infected with COVID-19 | [63] |
| 1st January-16th April 2020 | Retrospective study | China | 6001 PLWH, 35 coinfected with COVID-19 | RT-PCR, Magnetic Chemiluminescence Enzyme Immunoassay | Viral load, CD4+ count | 15 HIV/SARS-CoV-2 co-infected patients had severe illness with 2 deaths. Older age and discontinued cART were associated with severe illness and death | Results from the small number of HIV/SARS-CoV-2 coinfected cannot be generalized to the population | [35] |
| 3rd March-15th May 2020 | Retrospective cohort study | United States | 30 HIV patients, 90 control groups without HIV | RT-PCR | Viral load, CD4+ count, Lymphocyte count, LDH, D-dimer, Procalcitonin, CRP | No difference in the need for mechanical ventilation during hospitalization, length of stay or mortality between PLWH and non-PLWH who are co-infected with COVID-19 | Small sample size | [64] |
| March-April 2020 | Retrospective study | United Kingdom | 18 PLWH, 16 + with COVID-19 | RT-PCR | Viral load, CD4+ count | 3 out of 18 PLWH died | Small sample size | [65] |
| April 10, 2020 | Case Report | South Africa | 2 HIV+/COVID-19+ | RT-PCR | Oxygen saturation level, Arterial blood gas, X-ray, CT scan, Viral load, CD4+ scan | PLWH have a good outcome due to their impaired immune response | Small sample size | [66] |
| 1st March-30th June 2020 | Retrospective study | Italy | 31 HIV+/COVID-19+ | RT-PCR | Viral load, CD4+ count, Oxygen saturation level | Patients did not require ventilation and recovered 9 days after admission | Small sample size | [67] |
| Not stated | Case report | United Arab Emirates | 1 HIV+/COVID-19+, Kaposi + | RT-PCR | FBC, Viral load, T cell differential count, Renal function test, Blood culture, Respiratory pathogen panel, LDH, D-dimer, Fibrinogen, Ferritin Procalcitonin, CRP | Co-morbidities were associated with death | Small sample size | [68] |
| 12th March-23rd April 2020 | Cohort study | United States, New York | 4402 COVID-19+, 88 PLWH | RT-PCR | White blood count, creatinine, ALT, ferritin, IL-6, D-dimer, LDH, Procalcitonin, CRP, oxygen saturation level | There was frequent hospitalization among PLWH compared to non-PLWH. | Small sample size of PLWH | [11] |
| 20th March-30th April 2020 | Retrospective study | United States | 14 PLWH coinfected with COVID-19 | RT-PCR | X-ray, Viral load | 8 patients were hospitalized and 6 self-quarantined. There was no death | Small sample size | [69] |
| January-April 2020 | Cohort study | France | 30 HIV patients, 90 control groups without HIV | RT-PCR | CD4+ count, Viral load | 80% recovered from COVID-19 infection, 10% required ventilation, 6.7% died and 13.3% required hospitalization | Not reported | [70] |
| January-April 2020 | Observational prospective study | Spain | 51 HIV patients | RT-PCR | Viral load, CD4+ count, Full blood count, Renal function test, ALT, Procalcitonin, CRP, ferritin, IL-6, IL-12, LDH, D-dimer, X-ray, Oxygen saturation level | 4% of the HIV/SARS-CoV-2 individuals died | The small number of individuals prevented generalisation of results | [6] |
| 8th February 2020 | Case report | China | 2 HIV patients | RT-PCR | IL-6, procalcitonin, ferritin, CRP, Albumin, CD4+ count, Viral load, X-ray, Sars CoV2 abs test | Patients recovered. | Results were based on only two patients, and no follow-up was done due to limit resource | [71] |
| 2nd March-23rd April 2020 | Retrospective study | United States, New York | 21 HIV+, 2617 HIV- COVID-19+ | RT-PCR | FBC, procalcitonin, CRP, Troponin, D-dimer, ferritin, LDH, Creatinine, Creatinine phosphokinase, Respiratory rate, CD4+ count, Temperature, Blood pressure | Co-morbidity, higher inflammatory markers were associated with higher admission. All patients with comorbidity died | Lack of clinical data on participants | [72] |
| Small sample size | ||||||||
| 11th March-17th April 2020 | Retrospective study | Germany | 33 HIV participants | RT-PCR | Respiratory rate, CD4+ count, Viral load, Oxygen saturation | 3 of the patients died, 91% recovered and 76% mild cases | Small uncontrolled case series with limited follow up | [73] |
| January-9th March 2020 | Case series | Spain | 543 COVID-19 patients | RT-PCR | Viral load, CD4+ count, oxygen saturation, CRP, LDH, D-dimer, FBC | 4 out of 5 patients were cured by the end of the study | Small sample size | [74] |
| 5 HIV patients: 3 MSM, 2 transgenders | ||||||||
| 15th March-15th April 2020 | Case report | United States, New York | 31 PLWH infected with COVID-19 | RT-PCR | Vural load, CD4+ count, CRP, D-dimer, Ferritin, Procalcitonin, radiological findings | 8 patients died, and 21 recovered | Smaller sample size | [75] |
| There was no comparison with patients without HIV. | ||||||||
| March 2020-September 2021 | Case series | Tokyo, Japan | 17 HIV-COVID-19 patients | RT-PCR | CD4+ count, lymphocyte, CD8+ count | All patients recovered. No difference in CD4+ and CD8+ counts between onset of symptoms and after recovery | Small sample size | [28] |
| 2nd January–31st October 2020 | Observational retrospective monocentric cohort | Paris, France | 129 HIV individuals with COVID-19 | RT-PCR | Viral load, CD4+ count | Older age, higher BMI, diabetes, chronic kidney disease, transgender women were prone to disease severity with poor outcomes | Not all patients were confirmed to have COVID-19 by PCR. | [76] |
| January 2020- Not stated | Retrospective cohort study | Italy, Rome | 1647 hospitalized patients, 43 PLWH, 1605 non PLWH | RT-PCR | CD4+ count, Full blood count, Viral load, Potassium, CRP, D-dimer, Ferritin, oxygen saturation | There was less death among PLWH as compared to non-PLWH. | Small sample size | [77] |
| Analysis done at the study site cannot be generalized to other sites | ||||||||
| 29th March 2020 | Case Report | Korea | 1 HIV positive man | RT-PCR | CD4+ and CD8+ count, Viral load, Chest X-ray, CT scan, ESR and platelet count | Patient recovered | small sample size | [78] |
| May 2020 | Case Report | Brazil | 1 HIV positive woman | RT-PCR | CD4+ count, CD8+ count, Viral load, Chest X-ray, Oxygen saturation level | Patient recovered after a week | Small sample size | [79] |
| 1st march–9th June 2020 | Cohort study | Cape Town, South Africa | 3,460,932 public patients, 3978 HIV patients with COVID-19 | RT-PCR | CD4+ count, CD8+ count, Viral load | HIV and tuberculosis were associated with COVID-19 mortality | There was lack of data on co-morbidities and potential risk factors | [80] |
| 11th June–28th August 2020 | Observational case control study | Cape-Town, South Africa | 104 COVID-19 positive patients, 31 HIV/COVID-19 patients | RT-PCR, Neutralization assay, Flow cytometry | Cd4+ count, Viral load, LDH, Ferritin, D-dimer | 30 patients died | Analysis was not empowered to reproduce relationships between HIV and severity of COVID-19 | [81] |
| 1st June–1st October 2021 | Observation study | Guinea Bissau | 294 PLWH | COVID-19 IgM/IgG rapid test kit | - | Six deaths reported | The study population consisted only of patients on follow-up | [82] |
| 55 PLWH positive for SARS-CoV-2 | ||||||||
| 1st March–30th April 2020 | Observational prospective monocentric study | France | 54 PLWH coinfected with COVID-19 | RT-PCR | Viral load, CD4+ count, IL-6 | Male sex, age, ethnic origin, metabolic disorder was associated with severity of disease. 2 deaths were recorded | Study did not assess the risk linked to immune deficiency | [83] |
| 17th January–18th June 2020 | Prospective Observational study | United Kingdom | 115 HIV patients | RT-PCR | Full blood count, prothrombin time, Creatinine, CRP | 63% increased risk of day 28 mortality among PLWH hospitalized with COVID-19 compared to HIV negative | Risk factors for a COVID-19 related hospitalisation among PLWH, and the role of certain antiretroviral agents in modulating such risks were not addressed | [84] |
| 47,424 HIV negative patient | ||||||||
| Not stated | Prospective study | China | 1178 HIV patients, 8 co-infected | RT-PCR | CD4+ count, Viral loads, CT scan | Older ages were prone to get infected with COVID-19 | Small co-infection size | [85] |
| Not stated | Retrospective cohort study | Massachusetts, United States | 49,673 non PLWH, 404 PLWH | RT-PCR | CRP, LDH, ALT, AST, Bilirubin, Ferritin | PLWH had higher mortality at day 30 and were likely to be hospitalized that non PLWH. | Lack of clinical data | [86] |
| 9th March 2020–8th March 2020 | Retrospective study | Burkina Faso | 419 PLWH | RT-PCR, ELISA | PLWH on integrase inhibitors were more likely to be infected than PLWH on non-nucleoside inhibitors | Study could not investigate if COVID-19 natural infection may confer comparable antibody immunity among PLWH. | [87] | |
| June 2020–May 2021 | Prospective Cohort study | South Africa | 236 PLWH, 143 non-PLWH | RT-PCR. Flow cytometry | CD4+ and CD8+ count, Viral load, Full blood count | Higher disease severity was associated with low CD4+count and higher Neutrophil to lymphocyte ratio in first wave as compared to second wave | Limited information on HIV related immune perturbations influencing long-term immunity to SARS-CoV-2 infection | [88] |
| Until March 2022 | Cross-sectional study | South Africa | 600,00 PLWH | RT-PCR | CD4+count, Viral load | Mortality occurred in 5.7% of PLWH. Mortality was associated with lower recent CD4+ count, no evidence of ART usage, high viral load, and co-morbidities | Study did not assess the impact of prior SARS-CoV-2 infection on COVID-19 outcomes | [89] |
| Not stated | Case Report | Netherlands | A 38-year-old male HIV patient | RT-PCR | CD4+ count, Viral load, Chest X-ray | Patient was admitted with prolong COVID-19 infection with undiagnosed HIV and severe impaired cellular immunity | Small sample size | [90] |
| 1st March 2020–30th November, 2020 | Prospective Cohort study | United States | 1785 PLWH, 189,350 non-PLWH | RT-PCR | Viral load, CD4+ count | 15% were hospitalized and 5% died. Tenofovir was associated with reduction in clinical events | Results covered the first 9 months of the pandemic and did not include follow up during second wave | [36] |
| 1st June 2020–15th June 2020 | Prospective cohort study | Italy | 55 COVID-19 positive patients, 69 HIV patients negative for COVID-19 | RT-PCR, ELISA | CD4 and CD8+ counts, Viral load, Chest X-ray | 4 deaths were recorded | Small sample size prevents generalization of results | [91] |
| Age and number of co-morbidities were associated with death | ||||||||
| Not stated | Case report | New York, United States | A 54-year-old man | RT-PCR | CD4+ count, Biochemical test, Coagulation profile, Ferritin, Il-6, Procalcitonin, CK-MB, Chest X-ray | Patient recovered | Small sample size | [92] |
| February 2020–October 2021 | Retrospective study | Sweden | 64, 815 COVID-19 patients: 121 HIV positive | RT-PCR | Viral load, CD4+ count | 8% of PLWH died. HIV infection was not a risk factor for severe COVID-19 | Number of hospitalized PLWH were small therefore limited the power | [93] |
| 1st January 2020–31st December, 2020 | Retrospective study | Spain | 117,694 COVID-19: 234 HIV positive | RT-PCR | Comorbidity assessment | 9.4% mortality among PLWH. Advanced liver disease was a predictor of death | Overestimation of hospitalization among PLWH. | [94] |
| 10th March 2020–30th May 2020 | Retrospective cohort study | Israel | 23 PLWH coinfected with COVID-19 | RT-PCR | Full blood count, Viral load, CD4+ count | 13% of in-hospital death, 9% mechanical ventilation, and 9% intensive care unit admission were recorded | Small sample size | [95] |
| 15th February–31st May 2020 | Retrospective multicentre cohort study | Belgium | 16,000 HIV patients:101 COVID-19 patients | RT-PCR | CD4+ count, Viral load, CT scan | 46% of patients were hospitalized, and 9% of patients died. Older age, sub-Saharan patients and those on integrase inhibitor were associated with hospitalization | Small sample size | [96] |
| No comparison made with non-HIV patient | ||||||||
| 1st March 2020–15th December 2020 | Observational prospective cohort study | Spain | 13,142 followed up HIV patients: 749 COVID-19 positive | RT-PCR | Viral load, CD4+ and CD8+ count | 13 patients died. Chronic co-morbidities were associated with severe outcomes | Not all cohorts were tested for SARS-CoV-2 so incidence rate was not assessed. Data did not include information on smoking and BMI. | [97] |
| December 2021 | Brief report | South Africa | 45-year-old man | RT-PCR, Viral sequencing | Viral load, CD4+ count, Chest x-ray, IgG and IgA antibodies | Prolonged infection in HIV individuals may lead to evolution of SARS-CoV-2 lineages | Not reported | [98] |
| 10th March 2020–6th June 2020 | Retrospective matched cohort study | New York, United States | 853 PLWH and 1621 HIV negative patients | RT-PCR | Viral load, CD4+ count, FBC, Biochemical test, CRP, D-dimer, Ferritin, Procalcitonin, ESR, fibrinogen | Hospitalized PLWH and controls show no difference in-hospital death. Co-morbidities and inflammatory markers differ for each cohort upon hospitalization indicating different mechanisms leading to severe COVID-19 | Data from Medical record did not include a history of treatment for co-morbidities, which prevented accounting for severity or progression of these conditions | [99] |
| March 2020–September 2020 | Observation Retrospective cohort study | Brazil | 17,101 COVID-19 patients: 130 PLWH | R- PCR | Viral load, CD4+ count, full blood count, Kidney function test, Liver function test, Arterial pH, pO2, pCO2 | 27.9% mortality in PLWH in 2020. No difference in mortality among PLWH and non-PLWH in 2021 | Small sample size | [23] |
| March 2021–December 2021 | ||||||||
| 1st January 2020–21st May 2021 | Retrospective cohort study | United States | 1,446,913 COVID-19 patients: 3660 PLWH | RT-PCR | Viral load, CD4+ count | PLWH with immune dysfunction have greater risk for severe COVID-19 outcomes | There was less representation of admission practices and disease severity in medical records from study site | [100] |
| 1st March 2020–30th November 2020 | Retrospective cohort study | United States | 487 COVID-19 patients: 88 PLWH | RT-PCR | Viral load, CD4+ count | People living with HIV have a higher risk of COVID-19 diagnosis than those without HIV, but the outcomes are similar in both groups | Study could not implement routine SARS-CoV-2 screening to identify all patients with SARS-CoV-2 infection | [101] |
| 1st January 2020–8th May 2021 | Retrospective cohort study | United States | 1, 436, 622 COVID-19 patients: 13, 170 HIV patients | RT-PCR | CD4+ count, Viral load | A low CD4+ count was associated with all the adverse COVID-19 outcomes, while viral suppression was only associated with reduced hospitalisation | Though was a large sample size, this did not represent all the COVID-19 infections in the country | [102] |
| 1st March–31st December 2020 | Retrospective cohort study | Indonesia | 4134 PLWH | RT-PCR | Viral load, CD4+ count | 23 patients developed severe-critical COVID-19, and the mortality rate was 3.2% | The incidence of infection in the study population could not be assessed because not all participants were tested for SARS-CoV-2 | [103] |
| 342 PLWH with COVID-19 | ||||||||
| 28th January 2020 | Editorial | China | 61-year-old HIV man | RT-PCR | FBC, oxygen saturation, CT scan, CD4+ count | HIV infection need to be regarded as vulnerable group | Small sample size | [104] |
Summary of clinical outcomes on HIV and SARS-CoV-2 co-infection studies and their spatial distribution.
TABLE 2
| Sampling date | Study design | Study place | Study participants/Sample size | Assay type | Additional tests | Clinical outcomes/key findings | Limitations | References |
|---|---|---|---|---|---|---|---|---|
| 2nd March-15th April 2020 | brief report | United States | 72 HIV patients | RT-PCR | Viral load, CD4+ count, IL6, CRP, IL8, fibrinogen, D-dimer, TNF, IL-1B | High inflammatory markers and immune dysregulation were linked to death in PLWH. | Study was limited to 1 hospital. Complete HIV history was not available on all patients, and laboratory markers were obtained at the discretion of treating physicians | [17] |
| June-October 2020 | Prospective study | Iran | 155 HIV-1 patients | RT-PCR, Enzyme immunoassay | Viral load, CD4+ count, Hepatitis B, C, Tb test, | Higher anti-SARS-CoV-2 were reported in males than females | Screening and identification of HIV-1-infected individuals were limited due to COVID-19 lockdown | [29] |
| June-November 2020 | Longitudinal study | South Africa | 72 COVID-19 patients, 42 without HIV, 30 with HIV, 25 on ART | RT-PCR, Enzyme immunoassay (IgA, Igg, IgM), Microneutralization assay, Whole genome sequencing | CD4+ count, CD8+ count, Viral load | Antibody response among PLWH were comparable to those of non-PLWH. | Sample size small. There is a possibility of missing peak IgM response due to time of sampling | [12] |
| Only 16 out of 72 full genomes were sequenced | ||||||||
| January-June 2020 | retrospective study | Italy, Spain & Germany | 175 HIV patients | RT-PCR | Viral load, CD4+ count | Low CD4+ count was associated with high mortality rate in PLWH. | Only data on absolute CD4 T cells were available. Data on other lymphocyte subpopulations such as CD8 T cells were lacking | [105] |
| December 2020 | Case report | Spain | 1 HIV patient | RT-PCR, Whole Genome sequencing, Flow cytometry | Viral load, CD4+ count, full blood count, Computed Topography (CT) scan | T cell exhaustion was associated with severity of disease among PLWH. | Small sample size | [18] |
| Not stated | Case report | Italy | 1 HIV patient | RT-qPCR, flow cytometry, RT-PCR | CD4+ and CD8+ count, viral load, FBC, Arterial blood gas, Computed Topography scan | IFNα/β mRNAs and T cell activation were associated with severe pneumonia | Small sample size | [19] |
| 15th January-20th November 2020 | Prospective Cohort study | China | 18 PLWH, 185Non PLWH | RT-PCR, Immunochromatography assay | Lymphocyte count | Positive conversion rate of IgG was lower and quickly lost in PLWH compared to non-PLWH. | Lack of an antibody detection kit in the early days of the SARS-CoV-2 epidemic prevented early antibody testing | [30] |
| 20th March-15th June 2020 | Cohort study | Russia | 376 (171 ART experienced, 205 ART naïve) | RT-PCR, Flow cytometry, ELISA | Respiratory score | HIV ART-naïve was reported as a strong co-morbidity of severe COVID-19 | Not reported | [32] |
| 382 control groups | ||||||||
| 1st March-12th May 2020 | Observational study | Italy | 604 HIV participants | RT-PCR, Immunofluorescence, Microneutralization test, Flow cytometry, Elispot assay, ELISA | CT scan, Lymphocyte count, LDH, D-dimer, Fibrinogen, Ferritin | Adaptive cellular immune response correlated with disease severity | Small sample size makes it difficult to distinguish real effects from random variations thereby no definitive conclusions can be made | [20] |
| 11th February 2020 | Case report | China | 1 HIV patient | RT-PCR | CRP, LFT, LDH, FBC, oxygen saturation | Slower generation of antibodies was attributed to severity of disease | Small sample size | [31] |
| 6th March–11th September 2020 | Retrospective study | South Africa | 676 COVID-19 patients: 108 HIV | RT-PCR, ELISA | CD4+ count, Viral load, FBC, RFT, LFT, CRP, Troponin T, LDH, D-dimer, Troponin T, Ferritin, beta-d-glucan, procalcitonin | No significant difference in mortality between the HIV-positive and HIV- negative groups. HIV-positive patients who died were younger than the HIV-negatives | It was a single centre study, and so the data may not be generalized. some data capturing was retrospective, due to rapidly increasing patient numbers and staff shortages, and as a result some data were missing | [106] |
| 8th February 2020 | Case report | China | 2 HIV patients | RT-PCR | IL-6, procalcitonin, ferritin, CRP, Albumin, CD4+ count, Viral load, X-ray, Sars CoV2 abs test | Patients recovered | Small sample size | [71] |
| 8th June 2020–25th September, 2020 | Cross sectional study | South Africa | 126 HIV participants | RT-PCR, Flow cytometry, ELISA | CD4+ and CD8+ counts, Viral load | B cell responses were rapid but gave rise to lower affinity antibodies, less durable long-term memory, and reduced capacity to adapt to new variants | Study could not determine long-term effects of HIV on SARS-CoV-2 immunity, as new variants emerge | [27] |
| June–December 2020–1st wave | Longitudinal observational cohort study | South Africa | 25 HIV participants 1st wave | RT-PCR, Flow cytometry | Viral load, CD4+ count | Unsuppressed HIV infection impaired T cell responses to SARS-CoV-2 infection and diminishes T cell cross-recognition | Study did not examine relationship between CD8+ and CD19 subset at antigen-specific level due to sample limitations | [26] |
| January–June 2021- 2nd wave | 23 HIV participant 2nd wave | |||||||
| HIV negative 17 | ||||||||
| Not stated | Case report | Taiwan | A 38-year-old man | RT-PCR, ELISA, Virus neutralization assay | CRP, Viral load, CD4+ count, ALT, AST, Chest X-ray | Neutralizing antibody reached a plateau from 26th to 47th day onset but decreased on 157th day after symptoms | Small sample size | [107] |
| 4th March 2020 | Cross sectional study | China | 24 HIV patients: 21 had COVID-19 | RT-PCR | Ct scan, Chest X-ray, CRP, Procalcitonin, Viral load, CD4+ count, Full blood count, coagulation profile, Biochemical test | Reduction in T-cell number positively correlates with the serum levels of interleukin 6 (IL-6) and C-reactive protein (CRP) | Small sample size. Lack of detection of TCR zeta-chain expression | [30] |
| Not stated | Prospective cohort study | Zambia | 46 HIV negative patients | RT-PCR, Immuno-spot assay. Immunofluorescence assay, flow cytometry | CD4+ count, viral load | SARS-CoV-2-specific T cell immune responses may be delayed in individuals who are HIV +, even in those on antiretroviral therapy. There is no difference in SARS-CoV-2- specific humoral immunity between individuals who are HIV- and HIV+ | Small sample size limited study’s ability to elicit some of the differences that might exist between the sub- groups | [33] |
| 39 HIV positive patients | ||||||||
| 25th January 2020 | Brief Report | China | 38-year-old HIV man | RT-PCR, Chemiluminescence assay | FBC, CRP | Total Ab level was largely increased, and IgM remained at the peak level 1 week later, suggesting that the antibody responses against SARS-CoV-2 in this HIV-infected case was delayed | Studies did not to address the mechanism underlying the delayed antibody response to SARS-CoV-2 with a history of coinfection of HIV-1 infection | [108] |
| June 2020–August 2020 | Prospective cohort study | South Africa | 133 hospitalized patients: 95 COVID-19 patients (31 positive for HIV) | Flow cytometry, RT-PCR, electro chemiluminescent immunoassay | CD4+ count, Viral load, CRP, D-dimer, LDH, ferritin, WBC | SARS-CoV-2–specific CD4+ T cell attributes were associated with disease severity | Study could not use different approaches (such as the activation-induced markers assay) to confirm the inability of lymphopenia patients to mount a T cell response to SARS-CoV-2 | [21] |
| 30 non COVID-19 patients (with 13 positives for HIV) | Severe disease was characterized by poor polyfunctional potential, reduced proliferation capacity, and enhanced HLA-DR expression | |||||||
| 5th May 2020–22nd February, 2021 | Retrospective Cohort study | United States | 2464 PLWH: 283 COVID-19 positives | RT-PCR, ELISA, Luminex assay | Viral load, CD4+ count | SARS-CoV-2–specific humoral immune profiles among PLWH with obesity or lower nadir CD4+ T cell count was associated with worse outcomes | The study’s cross-sectional nature limits the ability to assess humoral repertoire changes over time in relation to COVID-19. Study was not able to control for statin use, given the ongoing nature of the trial | [109] |
| May 2020–October 2020 | Observational cohort study | United States, Peru | 43 PLWH, 330 non PLWH | RT-PCR. ELISA | Viral load, CD4+ count | Decreased SARS-CoV-2–specific antibodies among PLWH compared to non PLWH. | The median duration from diagnosis to enrolment was nearly 2 months, which did not fully represent the convalescent period | [34] |
| PLWH who recovered from COVID-19 had diminished immune responses and lacked an increase in SARS-CoV-2 antibodies | ||||||||
| Not stated | Prospective study | Barcelona, Spain | 50 patients, 11 PLWH, 39 non PLWH | RT-PCR, EliSpot immune assay, Fluorospot immune assay, ELISA | Oxygen saturation | PLWH developed a comparable short and long-term natural functional cellular and humoral immune response than non PLWH convalescent patients, which are highly influenced by the clinical severity of the COVID-19 infection | Patients with critical COVID-19 (requiring Mechanical ventilation) could not be obtained during the first wave of the pandemic and may be under-represented in this study | [110] |
| September–November 2020 | Observational cohort study | Italy | HIV with COVID-19: 30 | RT-PCR, micro-neutralization assay, ELISpot assay, | Viral load, CD4+ count, oxygen saturation | Significantly higher levels of IL-6, IL-8, and TNF-α in COVID-19 without HIV compared to HIV/COVID-19 patients were observed | Studies did not evaluate the persistence of these immunity and its ability to expand after exposure | [24] |
| HIV without COVID-19: 52 | ||||||||
| COVID-19 without HIV: 58 | ||||||||
| April-September, 2020 | cohort study | Italy | Young HIV patients | RT-qPCR, ELISA, Magnetic bead immunoassay, Geneplex assay, cytokine multiplex assay | CD4+ count, Liver function test, Renal function test, Clotting Profile | IL-10 could play a crucial role in the course of SARS-CoV-2 infection in HIV-positive individuals | Small sample size could lead to higher variability | [52] |
| 85 ART experienced control group 13 | ||||||||
| March 2020–September 2021 | Cohort study | United Kingdom | 47 HIV individuals, 24 confirmed COVID-19, 35 HIV negative | RT-PCR | Lymphocyte count, CD4+ count, CD8+ count, Spike IgG, N IgG antibodies, IFN-γ, TNF-α | Inadequate immune reconstitution on ART, could hinder immune response to SARS-CoV-2 | Study was not well powered | [25] |
Summary of immunological response on HIV and SARS-CoV-2 co-infection studies and their spatial distribution.
Legend: PLWH, people living with HIV; HIV, human immunodeficiency virus; CRP, C-reactive protein; FBC, full blood count; TNF, Tumour Necrotic factor; LFT, liver function test; RFT, renal function test; IL, interleukin; LDH, lactate dehydrogenase; RT PCR, Real time polymerase chain reaction; IgG, Immunoglobulin G; ALT, alanine transferase; AST, Aspartate Transferase; CT, scan, Computed topography scan; ESR, erythrocyte sedimentation rate; WBC, white blood cells; pCO2, Partial pressure of carbon dioxide; pO2, partial pressure of oxygen.
Thirty (38) studies reported the following risk factors as associated with severity of diseases (Table 1). This includes older age, higher BMI, male sex, deprivation, ethnicity, obesity, smoking, Tuberculosis, chronic kidney disease, higher inflammatory markers, diabetes, cardiovascular disease, lung cancer, African American, high viral load, low CD4+ count, high neutrophil-lymphocyte ratio, discontinued ART usage and some ART regimen. Twenty studies however indicated that clinical presentations among the co-infected were the same as the general population therefore there was low risk of disease severity (Table 1).
Twenty-five studies looked at immunological responses (Table 2), out of which four suggested that high inflammatory markers and immune dysregulation are linked to severity of disease and death among people who are coinfected with HIV/SARS-CoV-2 and are on ART, even though the ART is supposed to help with HIV viral suppression and immune reconstitution [17–22]. HIV/SARS-CoV-2 individuals with higher pro-inflammatory markers such as C-reactive protein (CRP), IL-8, IL-6 presented with disease severity and higher mortality than those who recovered [17]. Three other studies on co-infections linked reduction of T cell numbers to increased IL-6, IL-8, and CRP levels, causing a cytokine storm [23–25]. Among the co-infected individuals, unsuppressed HIV hampers T cell cross-recognition and responses to SARS-CoV-2 infection, and thereby leading to severe outcomes [26, 27]. The pre-symptom and post recovery CD4+, and CD8+ counts showed no significant difference between PLWH and HIV negative individuals who are infected with SARS-CoV-2 [28]. PLWH saw a brief decline in CD4+, and CD8+ counts during the acute phase of COVID-19 with the CD4+/CD8+ ratio remaining unchanged [11, 28].
Most of the studies were either retrospective or prospective with one time point sample collection, therefore, no subsequent CD4+ counts and viral loads to determine relationship with clinical outcomes. Two of the studies were longitudinal with one study investigating two waves of SARS-CoV-2 infection [26] and the other following up for a period of 3 months on HIV/SARS-CoV-2 patients [12]. Snyman et al., indicated in their study that anti-SARS-CoV-2 IgM, IgG, and IgA levels in non-HIV individuals and PLWH on full HIV suppression on ART have similar seroconversion rates [12]. The conversion rate of anti-SARS-CoV-2 IgG was lower and quickly lost in PLWH as compared to HIV negative persons who are SARS-CoV-2 positive [29–31]. Three of the studies indicated that slower generation of anti SARS CoV2 antibodies were attributed to increased COVID-19 severity among PLWH [32–34].
Discussion
We conducted a scoping review to assess specific COVID-19 clinical outcomes and immune response in patients with human immunodeficiency virus (PLWH) and identify gaps. Hospitalisation risk, intensive care unit admission, mechanical ventilation and mortality were the four categories identified as clinical outcomes. Our review showed varied reports on risk of hospitalisation, ICU admission, mechanical ventilation and mortality in cohort studies, case series, and case reports. PLWH who died exhibited higher levels of soluble immune activation and inflammation markers, which are linked to disease severity in COVID-19 [22]. Individuals with non-suppressed HIV viremia have reported lower levels of antibodies against SARS-CoV-2 in their humoral response [35]. Some studies however, associated low risk of hospitalization and death to Tenofovir usage as compared to those on other regimen [35–37].
Immune response to SARS-CoV-2 infection among PLWH on ART
ART does not eradicate HIV completely but significantly reduces morbidity and mortality associated with the virus [38]. ART may also reduce the severity of COVID-19 through immunological reconstitution, although these effects have not yet been confirmed [10, 36, 39]. PLWH with mild COVID-19 presentation, in the presence of high proinflammatory markers, suggested that certain antiretroviral drugs were protective against severity of COVID-19 disease [20]. A study in Russia among 376 HIV/COVID-19 patients (171 without ART and 205 with ART) suggested that elevated anti-inflammatory markers such as IL-10 and TGFβ, reduced CD4+/CD8+ cell ratios led to an increase in exhausted T cells in ART naïve patients. This led to Adverse Respiratory Distress Syndrome among the ART naïve group [32]. Sharov also reiterated that in the presence of uninterrupted ART, HIV patients do not progress to severe SARS-CoV-2 infection [32]. Other studies hypothesized that specific ART (NRTIs, NNRTIs and PI) predisposes to severe COVID-19 but no conclusive findings have been made because of studies involving smaller sample size and inconsistent cases and reports [40, 41].
Signaling pathway of HIV/SARS-CoV-2 coinfection
Viral infections interact mainly with the activated Signal Transducer and Activators of Transcription 1, 2, and 3 (STAT1, STAT2 and STAT3) to release pro-inflammatory cytokines to eliminate viruses [42]. The IL-6-JAK-STAT3 axis is significantly linked to the onset of severe COVID-19 [43, 44]. The dimerized epidermal growth factor receptor (EGFR) can tyrosine-phosphorylate STAT3, which is elevated in cases of acute lung injury [45] and in cases where STAT1 is lacking [46, 47]. As a result, in COVID-19, EGFR signalling may develop into a different pathway that stimulates STAT3 when lung damage occurs, and SARS-CoV-2 infection significantly reduces IFN-I production [48]. This aberrant transcriptional rewiring towards STAT3 may lead to the symptoms most typically reported in hospitalised COVID-19 patients: fast coagulopathy/thrombosis, proinflammatory conditions, profibrotic state, and T cell lymphopenia [49].
Some HIV proteins have been reported to inhibit effective IFNα signalling by degrading certain components of the JAK/STAT signalling pathway like STAT1 and STAT3 [50]. The impaired JAK/STAT signalling pathway is however restored in the presence of uninterrupted combined Antiretroviral therapy (cART) for more than 6 months [51]. Per our search, we found one study available on HIV/COVID-19 signalling pathway that investigated STAT3 but did not look at other STAT pathways [52], and therefore creates a gap that needs to be researched. Understanding the viral co-infection, immune response, and signalling pathway dynamics will help identify particulate markers that predisposes to severity of disease.
Oxidative stress responses among HIV/SARS-CoV-2 coinfection
Hyperactivation of STAT3 affect various biological and physiological functions, leading to oxidative stress (OxS) and poor prognosis of disease [22]. Oxidative stress (OxS) comes about by accumulating reactive oxygen and nitrogen species, which are free radicals that causes injury to organs. Under physiological conditions, these OxS are wiped out by antioxidants especially glutathione (GSH) [53]. Glutathione are endogenous intracellular antioxidants that neutralizes free radical released due to oxidative stress [54]. Deficiency in GSH however, leads to high levels of OxS due to compromised antioxidant defences [55]. Oxidative stress has been studied in HIV or SARS-CoV 2 alone with higher levels reported in each disease [55–58]. There is however scanty information on oxidative stress among HIV/COVID-19 patients, hence the need to investigate if the presence of ART usage affects oxidative stress response.
Limitations
There is lack of information on cellular immunity in other hCoVs apart from COVID-19 co-infection. Cytokine have been studied extensively in HIV or COVID-19 alone but not as a co-infection. The oxidative stress levels among HIV/SARS-CoV-2 co-infection are yet to be studied although research has been done for other co-morbidities or co-infections.
Conclusion
This study highlights the paucity of clinical and immunological data on HIV/SARS-CoV-2 co-infection in sub-Saharan Africa, even though this region has the highest HIV prevalence. Review shows conflicting reports on severity of the co-infection. HIV/SARS-CoV-2 severity and outcomes appear to be worse, when coexisting age-related comorbidities and CD4 + T-cell depletion is present. Discontinued or no evidence of ART usage have also been shown to increase disease severity, which needs to be studied further to ascertain its authenticity.
CD4+ T cell lymphopenia in both diseases is influenced by various mechanisms including direct attacks, immune activation, and redistribution of CD4+ T cells. Cytokines investigation will help identify markers that are implicated in disease severity among HIV/SARS-CoV-2 patients. Further investigation is needed to confirm co-infection-associated cytokines and/or immunological markers to SARS-CoV-2 in PLWH.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
Study conception was performed by EA, EB, ET, EP, KT, and OQ. Original draft preparation was performed by EA, PA, LA, and MA-P. Methodology was performed by EA, PA, LA, and MA-P. EB, ET, EP, KT, and OQ critically reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded in part by the Fogarty International Center of National Institute of Alcohol Abuse and Alcoholism of the National Institutes of Health (NIH) [D43 TW011526], the World Bank African Centres of Excellence grant [WACCBIP+NCDs: Awandare], and the Science for Africa Foundation to Developing Excellence in Leadership, Training and Science in Africa (DELTAS Africa) programme [DEL-22-014] with support from Wellcome and the UK Foreign, Commonwealth and Development Office (FCDO) which is part of the EDCPT2 programme supported by the European Union. The content of the research is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, World Bank, Wellcome Trust, or the FCDO.
Acknowledgments
The authors acknowledge the support of the West African Centre for Cell Biology of Infectious Pathogens (WACCBIP) and the HIVComRT.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
SchoutenJWitFWStolteIGKootstraNAvan der ValkMGeerlingsSEet alCross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis (2014) 59(12):1787–97. 10.1093/cid/ciu701
2.
ÖnenNFOvertonETSeyfriedWStummERSnellMMondyKet alAging and HIV infection: a comparison between older HIV-infected persons and the general population. HIV Clin trials (2010) 11(2):100–9. 10.1310/hct1102-100
3.
BrownTTGuaraldiG. Multimorbidity and burden of disease. Interdiscip Top Gerontol Geriatr (2017) 42:59–73. 10.1159/000448544
4.
KanwuguONAdadiP. HIV/SARS‐CoV‐2 coinfection: a global perspective. J Med Virol (2021) 93(2):726–32. 10.1002/jmv.26321
5.
WHO. COVID-19 weekly epidemiological update. 134 edn (2023).
6.
VizcarraPPérez-ElíasMJQueredaCMorenoAVivancosMJDrondaFet alDescription of COVID-19 in HIV-infected individuals: a single-centre, prospective cohort. The lancet HIV (2020) 7(8):e554–e564. 10.1016/s2352-3018(20)30164-8
7.
CollinsLFMoranCAOliverNTMoannaALahiriCDColasantiJAet alClinical characteristics, comorbidities and outcomes among persons with HIV hospitalized with coronavirus disease 2019 in Atlanta, Georgia. AIDS (London, England) (2020) 34(12):1789–94. 10.1097/qad.0000000000002632
8.
CalzaLBonITadoliniMBorderiMColangeliVBadiaLet alCOVID-19 in patients with HIV-1 infection: a single-centre experience in northern Italy. Infection (2021) 49(2):333–7. 10.1007/s15010-020-01492-7
9.
HartleyDMPerencevichEN. Public health interventions for COVID-19: emerging evidence and implications for an evolving public health crisis. Jama (2020) 323(19):1908–9. 10.1001/jama.2020.5910
10.
Del AmoJPoloRMorenoSDíazAMartínezEArribasJRet alAntiretrovirals and risk of COVID-19 diagnosis and hospitalization in HIV-positive persons. Epidemiology (Cambridge, Mass.) (2020) 31(6):e49–e51. 10.1097/ede.0000000000001235
11.
SigelKSwartzTGoldenEParanjpeISomaniSRichterFet alCoronavirus 2019 and people living with human immunodeficiency virus: outcomes for hospitalized patients in New York City. Clin Infect Dis (2020) 71(11):2933–8. 10.1093/cid/ciaa880
12.
SnymanJHwaSHKrauseRMuemaDReddyTGangaYet alSimilar antibody responses against severe acute respiratory syndrome coronavirus 2 in individuals living without and with human immunodeficiency virus on antiretroviral therapy during the first South African infection wave. Clin Infect Dis (2022) 75(1):e249–e256. 10.1093/cid/ciab758
13.
BhaskaranKRentschCTMacKennaBSchultzeAMehrkarABatesCJet alHIV infection and COVID-19 death: a population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. The lancet HIV (2021) 8(1):e24–e32. 10.1016/s2352-3018(20)30305-2
14.
WatersLJPozniakAL. COVID-19 death in people with HIV: interpret cautiously. The Lancet HIV (2021) 8(1):e2–e3. 10.1016/s2352-3018(20)30332-5
15.
ScagnolariCAntonelliG. Type I interferon and HIV: subtle balance between antiviral activity, immunopathogenesis and the microbiome. Cytokine Growth Factor Rev (2018) 40:19–31. 10.1016/j.cytogfr.2018.03.003
16.
ZhouZRenLZhangLZhongJXiaoYJiaZet alHeightened innate immune responses in the respiratory tract of COVID-19 patients. Cell host & microbe (2020) 27(6):883–90. 10.1016/j.chom.2020.04.017
17.
HoH-e.PelusoMJMargusCMatias LopesJPHeCGaisaMMet alClinical outcomes and immunologic characteristics of coronavirus disease 2019 in people with human immunodeficiency virus. J Infect Dis (2021) 223(3):403–8. 10.1093/infdis/jiaa380
18.
ÁlvarezHRuiz-MateosEJuiz-GonzálezPMVitalléJViéitezIVázquez-FriolMCet alSARS-CoV-2 evolution and spike-specific CD4+ T-cell response in persistent COVID-19 with severe HIV immune suppression. Microorganisms (2022) 10(1):143. 10.3390/microorganisms10010143
19.
d’EttorreGRecchiaGRidolfiMSiccardiGPinacchioCInnocentiGPet alAnalysis of type I IFN response and T cell activation in severe COVID-19/HIV-1 coinfection: a case report. Medicine (2020) 99(36):e21803. 10.1097/md.0000000000021803
20.
MondiACiminiEColavitaFCicaliniSPinnettiCMatusaliGet alCOVID‐19 in people living with HIV: clinical implications of dynamics of the immune response to SARS‐CoV‐2. J Med Virol (2021) 93(3):1796–804. 10.1002/jmv.26556
21.
RiouCdu BruynEStekCDaroowalaRGoliathRTAbrahamsFet alRelationship of SARS-CoV-2-specific CD4 response to COVID-19 severity and impact of HIV-1 and tuberculosis coinfection. J Clin Invest (2021) 131(12):e149125. 10.1172/jci149125
22.
RuanQYangKWangWJiangLSongJ. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med (2020) 46(5):846–8. 10.1007/s00134-020-05991-x
23.
SalesTLSSouza-SilvaMVRDelfino-PereiraPNevesJVBSaciotoMFAssisVCet alCOVID-19 outcomes in people living with HIV: peering through the waves. Clinics (2023) 78:100223. 10.1016/j.clinsp.2023.100223
24.
VergoriABoschiniANotariSLorenziniPCastillettiCColavitaFet alSARS-CoV-2 specific immune response and inflammatory profile in advanced HIV-infected persons during a COVID-19 outbreak. Viruses (2022) 14(7):1575. 10.3390/v14071575
25.
AlrubayyiAGea-MallorquíETouizerEHameiri-BowenDKopycinskiJCharltonBet alCharacterization of humoral and SARS-CoV-2 specific T cell responses in people living with HIV. Nat Commun (2021) 12(1):5839. 10.1038/s41467-021-26137-7
26.
NkosiTNdhlovuZMChasaraCPapadopoulosAONguniTLKarimFet alUnsuppressed HIV infection impairs T cell responses to SARS-CoV-2 infection and abrogates T cell cross-recognition. Elife (2022) 11:e78374. 10.7554/elife.78374
27.
KrauseRSnymanJShi-HsiaHMuemaDKarimFGangaYet alHIV skews the SARS-CoV-2 B cell response towards an extrafollicular maturation pathway. Elife (2022) 11:e79924. 10.7554/eLife.79924
28.
AdachiESaitoMNagaiHIkeuchiKKogaMTsutsumiTet alTransient depletion of T cells during COVID‐19 and seasonal influenza in people living with HIV. J Med Virol (2022) 94(5):1789–91. 10.1002/jmv.27543
29.
GarshasbiSBokharaei-SalimFKhanalihaKKianiSJKalantariSJamshidi MakianiMet alSARS-CoV-2 infection in Iranian people living with human immunodeficiency virus-1 infection. Jundishapur J Microbiol (2022) 15(1). 10.5812/jjm.121929
30.
LiuYXiaoYWuSMarleyGMingFWangXet alPeople living with HIV easily lose their immune response to SARS-CoV-2: result from a cohort of COVID-19 cases in Wuhan, China. BMC Infect Dis (2021) 21(1):1029–7. 10.1186/s12879-021-06723-2
31.
WangMLuoLBuHXiaH. One case of coronavirus disease 2019 (COVID-19) in a patient co-infected by HIV with a low CD4+ T-cell count. Int J Infect Dis (2020) 96:148–50. 10.1016/j.ijid.2020.04.060
32.
SharovKS. HIV/SARS-CoV-2 co-infection: T cell profile, cytokine dynamics and role of exhausted lymphocytes. Int J Infect Dis (2021) 102:163–9. 10.1016/j.ijid.2020.10.049
33.
NgalamikaOLidengeSJMukasineMCKawimbeMKamanziPNgowiJRet alSARS-CoV-2-specific T cell and humoral immunity in individuals with and without HIV in an African population: a prospective cohort study. Int J Infect Dis (2023) 127:106–15. 10.1016/j.ijid.2022.12.009
34.
SchusterDJKarunaSBrackettCWesleyMLiSSEiselNet alLower SARS-CoV-2–specific humoral immunity in people living with HIV-1 recovered from nonhospitalized COVID-19. JCI insight (2022) 7(21):e158402. 10.1172/jci.insight.158402
35.
HuangJXieNHuXYanHDingJLiuPet alEpidemiological, virological and serological features of coronavirus disease 2019 (COVID-19) cases in people living with human immunodeficiency virus in Wuhan: a population-based cohort study. Clin Infect Dis (2021) 73(7):e2086–e2094. 10.1093/cid/ciaa1186
36.
LeaANLeydenWASofryginOMarafinoBJSkarbinskiJNapravnikSet alHuman immunodeficiency virus status, Tenofovir exposure, and the risk of poor coronavirus disease 19 outcomes: real-world analysis from 6 United States cohorts before vaccine rollout. Clin Infect Dis (2023) 76(10):1727–34. 10.1093/cid/ciad084
37.
Del AmoJPoloRMorenoSJarrínIHernánMA. SARS-CoV-2 infection and coronavirus disease 2019 severity in persons with HIV on antiretroviral treatment. Aids (2022) 36(2):161–8. 10.1097/qad.0000000000003132
38.
LohseNObelN. Update of survival for persons with HIV infection in Denmark. Ann Intern Med (2016) 165(10):749–50. 10.7326/l16-0091
39.
SuwanwongseKShabarekN. Clinical features and outcome of HIV/SARS‐CoV‐2 coinfected patients in the Bronx, New York city. J Med Virol (2020) 92(11):2387–9. 10.1002/jmv.26077
40.
CostenaroPMinottiCBarbieriEGiaquintoCDonàD. SARS‐CoV‐2 infection in people living with HIV: a systematic review. Rev Med Virol (2021) 31(1):1–12. 10.1002/rmv.2155
41.
MirzaeiHMcFarlandWKaramouzianMSharifiH. COVID-19 among people living with HIV: a systematic review. AIDS Behav (2021) 25:85–92. 10.1007/s10461-020-02983-2
42.
LuoYAlexanderMGadinaMO’SheaJJMeylanFSchwartzDM. JAK-STAT signaling in human disease: from genetic syndromes to clinical inhibition. J Allergy Clin Immunol (2021) 148(4):911–25. 10.1016/j.jaci.2021.08.004
43.
Giamarellos-BourboulisEJNeteaMGRovinaNAkinosoglouKAntoniadouAAntonakosNet alComplex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell host & microbe (2020) 27(6):992–1000. 10.1016/j.chom.2020.04.009
44.
HiranoTMurakamiM. COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity (2020) 52(5):731–3. 10.1016/j.immuni.2020.04.003
45.
FiniganJHDowneyGPKernJA. Human epidermal growth factor receptor signaling in acute lung injury. Am J Respir Cel Mol Biol (2012) 47(4):395–404. 10.1165/rcmb.2012-0100tr
46.
VenkataramanTColemanCMFriemanMB. Overactive epidermal growth factor receptor signaling leads to increased fibrosis after severe acute respiratory syndrome coronavirus infection. J Virol (2017) 91(12):e00182-17. 10.1128/jvi.00182-17
47.
YangLXuJGuoLGuoTZhangLFengLet alPorcine epidemic diarrhea virus-induced epidermal growth factor receptor activation impairs the antiviral activity of type I interferon. J Virol (2018) 92(8):e02095-17. 10.1128/jvi.02095-17
48.
HadjadjJYatimNBarnabeiLCorneauABoussierJSmithNet alImpaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science (2020) 369(6504):718–24. 10.1126/science.abc6027
49.
MatsuyamaTKubliSPYoshinagaSKPfefferKMakTW. An aberrant STAT pathway is central to COVID-19. Cel Death Differ (2020) 27(12):3209–25. 10.1038/s41418-020-00633-7
50.
GarganSAhmedSMahonyRBannanCNapoletanoSO'FarrellyCet alHIV-1 promotes the degradation of components of the type 1 IFN JAK/STAT pathway and blocks anti-viral ISG induction. EBioMedicine (2018) 30:203–16. 10.1016/j.ebiom.2018.03.006
51.
LiuM-QZhaoMKongWHTangLWangFZhuZRet alCombination antiretroviral therapy (cART) restores HIV-1 infection-mediated impairment of JAK-STAT signaling pathway. Oncotarget (2017) 8(14):22524–33. 10.18632/oncotarget.15121
52.
VanettiCTrabattoniDStracuzziMAmendolaAFappaniCRubinacciVet alImmunological characterization of HIV and SARS-CoV-2 coinfected young individuals. Cells (2021) 10(11):3187. 10.3390/cells10113187
53.
BallatoriNKranceSMNotenboomSShiSTieuKHammondCL. Glutathione dysregulation and the etiology and progression of human diseases. bchm (2009) 390:191–214. 10.1515/bc.2009.033
54.
YanYYangYWangFRenHZhangSShiXet alClinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res Care (2020) 8(1):e001343. 10.1136/bmjdrc-2020-001343
55.
KumarPOsahonOVidesDBHananiaNMinardCGSekharRV. Severe glutathione deficiency, oxidative stress and oxidant damage in adults hospitalized with COVID-19: implications for GlyNAC (glycine and N-acetylcysteine) supplementation. Antioxidants (2021) 11(1):50. 10.3390/antiox11010050
56.
PaceGWLeafCD. The role of oxidative stress in HIV disease. Free Radic Biol Med (1995) 19(4):523–8. 10.1016/0891-5849(95)00047-2
57.
RepettoMReidesCGomez CarreteroMLCostaMGriembergGLlesuyS. Oxidative stress in blood of HIV infected patients. Clinica Chim Acta (1996) 255(2):107–17. 10.1016/0009-8981(96)06394-2
58.
Ntyonga-PonoM-P. COVID-19 infection and oxidative stress: an under-explored approach for prevention and treatment?Pan Afr Med J (2020) 35(2):12. 10.11604/pamj.2020.35.2.22877
59.
SassetLDi MecoECavinatoSCattelanAM. Coinfection of severe acute respiratory syndrome coronavirus 2 and HIV in a teaching hospital: still much to learn. AIDS (2020) 34(11):1694–6. 10.1097/qad.0000000000002609
60.
TesorieroJMSwainCAEPierceJLZamboniLWuMHoltgraveDRet alCOVID-19 outcomes among persons living with or without diagnosed HIV infection in New York State. JAMA Netw open (2021) 4(2):e2037069. 10.1001/jamanetworkopen.2020.37069
61.
DandachiDGeigerGMontgomeryMWKarmen-TuohySGolzyMAntarAARet alCharacteristics, comorbidities, and outcomes in a multicenter registry of patients with human immunodeficiency virus and coronavirus disease 2019. Clin Infect Dis (2021) 73(7):e1964–e1972. 10.1093/cid/ciaa1339
62.
GervasoniCMeravigliaPRivaAGiacomelliAOreniLMinisciDet alClinical features and outcomes of patients with human immunodeficiency virus with COVID-19. Clin Infect Dis (2020) 71(16):2276–8. 10.1093/cid/ciaa579
63.
HuYMaJHuangHVermundSH. Coinfection with HIV and SARS-CoV-2 in Wuhan, China: a 12-person case series. J acquired immune deficiency syndromes (2020) 85(1):1–5. 10.1097/QAI.0000000000002424
64.
StoeckleKJohnstonCDJannat-KhahDPWilliamsSCEllmanTMVoglerMAet alCOVID-19 in hospitalized adults with HIV. Open Forum Infect Dis (2020) 7:ofaa327. Oxford University Press US. 10.1093/ofid/ofaa327
65.
MadgeSBarberTJHunterABhaganiSLipmanMBurnsF. Descriptive account of 18 adults with known HIV infection hospitalised with SARS-CoV-2 infection. Sex Transm Infections (2021) 97(5):392–3. 10.1136/sextrans-2020-054660
66.
MithaMMaharajANyamandeK. People living with HIV and COVID-19: a report on 2 clinical cases from South Africa. Afr J Thorac Crit Care Med (2020) 26(2):59–60. 10.7196/ajtccm.2020.v26i2.078
67.
CalzaLBonIBorderiMColangeliVBorioniAReMCet alCOVID-19 outcomes in patients with uncontrolled HIV-1 infection. JAIDS J Acquired Immune Deficiency Syndromes (2021) 86(1):e15–e17. 10.1097/qai.0000000000002537
68.
QasimAMansourMKousaOAwadDAbuhazeemBMillnerPet alA case of coronavirus disease 2019 in acquired immunodeficiency syndrome patient: a case report and review of the literature. Intractable Rare Dis Res (2020) 9(4):256–9. 10.5582/irdr.2020.03081
69.
GudipatiSBrarIMurraySMcKinnonJEYaredNMarkowitzN. Descriptive analysis of patients living with HIV affected by COVID-19. JAIDS J Acquired Immune Deficiency Syndromes (2020) 85(2):123–6. 10.1097/qai.0000000000002450
70.
IserniaVJuliaZLe GacSBachelardALandmanRLarivenSet alSARS-COV2 infection in 30 HIV-infected patients followed-up in a French University Hospital. Int J Infect Dis (2020) 101:49–51. 10.1016/j.ijid.2020.09.1436
71.
ZhangJ-CYuXHDingXHMaHYCaiXQKangSCet alNew HIV diagnoses in patients with COVID-19: two case reports and a brief literature review. BMC Infect Dis (2020) 20(1):771–10. 10.1186/s12879-020-05480-y
72.
Karmen-TuohySCarlucciPMZervouFNZacharioudakisIMRebickGKleinEet alOutcomes among HIV-positive patients hospitalized with COVID-19. JAIDS J Acquired Immune Deficiency Syndromes (2020) 85:6–10. 10.1097/qai.0000000000002423
73.
HärterGSpinnerCDRoiderJBickelMKrznaricIGrunwaldSet alCOVID-19 in people living with human immunodeficiency virus: a case series of 33 patients. Infection (2020) 48:681–6. 10.1007/s15010-020-01438-z
74.
BlancoJLAmbrosioniJGarciaFMartínezESorianoAMallolasJet alCOVID-19 in patients with HIV: clinical case series. The lancet HIV (2020) 7(5):e314–e316. 10.1016/s2352-3018(20)30111-9
75.
ShalevNSchererMLaSotaEDAntoniouPYinMTZuckerJet alClinical characteristics and outcomes in people living with human immunodeficiency virus hospitalized for coronavirus disease 2019. Clin Infect Dis (2020) 71(16):2294–7. 10.1093/cid/ciaa635
76.
BachelardASautereauADigumberMIserniaVPhungBLehurACet alRisk factors associated with severe/critical COVID-19 in people living with HIV-1. Int J Infect Dis (2022) 122:152–4. 10.1016/j.ijid.2022.05.055
77.
GagliardiniRVergoriALorenziniPCicaliniSPinnettiCMazzottaVet alCharacteristics and outcomes of COVID-19-related hospitalization among PLWH. J Clin Med (2022) 11(6):1546. 10.3390/jcm11061546
78.
KimJ-YKimJMPeckKR. The first case of an HIV patient diagnosed with COVID-19 in Korea. J Korean Med Sci (2020) 35(39):e358. 10.3346/jkms.2020.35.e358
79.
CipolatMMSprinzE. COVID-19 pneumonia in an HIV-positive woman on antiretroviral therapy and undetectable viral load in Porto Alegre, Brazil. Braz J Infect Dis (2020) 24:455–7. 10.1016/j.bjid.2020.07.009
80.
DaviesM-A. HIV and risk of COVID-19 death: a population cohort study from the Western Cape Province, South Africa. MedRxiv (2020). Available from: https://doi.org/10.1101/2020.07.02.20145185.
81.
Du BruynEStekCDaroowalaRSaid-HartleyQHsiaoMSchaferGet alEffects of tuberculosis and/or HIV-1 infection on COVID-19 presentation and immune response in Africa. Nat Commun (2023) 14(1):188. 10.1038/s41467-022-35689-1
82.
DutschkeAWejseCNanqueJMedinaCHøngeBJespersenSet alSARS-CoV-2 seroprevalence among people living with HIV in Guinea–Bissau. Public Health (2022) 209:36–8. 10.1016/j.puhe.2022.05.017
83.
EtienneNKarmochkineMSlamaLPavieJBatisseDUsubillagaRet alHIV infection and COVID-19: risk factors for severe disease. AIDS (London, England) (2020) 34(12):1771–4. 10.1097/qad.0000000000002651
84.
GerettiAMStockdaleAJKellySHCevikMCollinsSWatersLet alOutcomes of COVID-19 related hospitalisation among people with HIV in the ISARIC WHO Clinical Characterisation Protocol UK Protocol: prospective observational study. MedRxiv (2020). Available from: https://doi.org/10.1101/2020.08.07.20170449.
85.
GuoWMingFDongYZhangQZhangXMoPet alA survey for COVID-19 among HIV/AIDS patients in two districts of Wuhan, China (2020). Available from: http://dx.doi.org/10.2139/ssrn.3550029.
86.
HadiYBNaqviSFKupecJTSarwariAR. Characteristics and outcomes of COVID-19 in patients with HIV: a multicentre research network study. AIDS (London, England) (2020) 34(13):F3–F8. 10.1097/qad.0000000000002666
87.
KaboréODPodaAOuattaraCAMichodigniFNBelemAASawadogoYet alSeroprevalence of SARS-CoV-2 IgG and associated factors among people living with HIV over the first 12 months following the outbreak of COVID-19 in Burkina Faso, a sub-Saharan African country. Plos one (2023) 18(6):e0286665. 10.1371/journal.pone.0286665
88.
KarimFGazyICeleSZunguYKrauseRBernsteinMet alHIV status alters disease severity and immune cell responses in Beta variant SARS-CoV-2 infection wave. Elife (2021) 10:e67397. 10.7554/eLife.67397
89.
KassanjeeRDaviesMNgwenyaOOsei‐YeboahRJacobsTMordenEet alCOVID‐19 among adults living with HIV: correlates of mortality among public sector healthcare users in Western Cape, South Africa. J Int AIDS Soc (2023) 26(6):e26104. 10.1002/jia2.26104
90.
KetelsTGisolfJClaassenMSwaninkCvan LochemEMoonenLet alShort communication: prolonged COVID-19 infection in a patient with newly diagnosed HIV/AIDS. AIDS Res Hum retroviruses (2022) 38(5):399–400. 10.1089/aid.2021.0145
91.
MaggioloFZoboliFArosioMValentiDGuarneriDSangiorgioLet alSARS‐CoV‐2 infection in persons living with HIV: a single center prospective cohort. J Med Virol (2021) 93(2):1145–9. 10.1002/jmv.26352
92.
MahmoodKRashedEROliverosEChauVQHermleTJacobsSet alPredisposition or protection? COVID-19 in a patient on LVAD support with HIV/AIDS. JACC: Case Rep (2020) 2(9):1337–41. 10.1016/j.jaccas.2020.05.015
93.
MöllerIKGisslénMWagnerPSparénPCarlanderC. COVID‐19 hospitalization outcomes in adults by HIV status; a nation‐wide register‐based study. HIV Med (2023) 24:1045–55. 10.1111/hiv.13515
94.
Moreno-TorresVde MendozaCMartínez-UrbistondoMMillsPTreviñoAde la FuenteSet alPredictors of in-hospital mortality in HIV-infected patients with COVID-19. QJM: Int J Med (2023) 116(1):57–62. 10.1093/qjmed/hcac215
95.
NagarakantiSROkohAKGrinbergSBishburgE. Clinical outcomes of patients with COVID‐19 and HIV coinfection. J Med Virol (2021) 93(3):1687–93. 10.1002/jmv.26533
96.
NasreddineRFlorenceEMoutschenMYombiJGoffardJDerdelinckxIet alClinical characteristics and outcomes of COVID‐19 in people living with HIV in Belgium: a multicenter, retrospective cohort. J Med Virol (2021) 93(5):2971–8. 10.1002/jmv.26828
97.
NomahDKReyes-UrueñaJDíazYMorenoSAceitonJBrugueraAet alSociodemographic, clinical, and immunological factors associated with SARS-CoV-2 diagnosis and severe COVID-19 outcomes in people living with HIV: a retrospective cohort study. The Lancet HIV (2021) 8(11):e701–e710. 10.1016/s2352-3018(21)00240-x
98.
PetersJLFallALangermanSDEl AsmarMNakazawaMMustaphaAet alProlonged severe acute respiratory syndrome coronavirus 2 Delta variant shedding in a patient with AIDS: case report and review of the literature. Open Forum Infect Dis (2022) 9:ofac479. Oxford University Press US. 10.1093/ofid/ofac479
99.
RosenthalEMRosenbergESPattersonWFergusonWPGonzalezCDeHovitzJet alFactors associated with SARS-CoV-2-related hospital outcomes among and between persons living with and without diagnosed HIV infection in New York State. PLoS One (2022) 17(5):e0268978. 10.1371/journal.pone.0268978
100.
SunJPatelRCZhengQMadhiraVOlexALIslamJYet alCOVID-19 disease severity among people with HIV infection or solid organ transplant in the United States: a nationally-representative, multicenter, observational cohort study. Medrxiv (2021). Avaialble from: https://doi.org/10.1101/2021.07.26.21261028.
101.
TangMEGaufinTAnsonRZhuWMathewsWCachayER. People with HIV have a higher risk of COVID‐19 diagnosis but similar outcomes to the general population. HIV Med (2022) 23(10):1069–77. 10.1111/hiv.13312
102.
YangYIwasakiA. Impact of chronic HIV infection on SARS-CoV-2 infection, COVID-19 disease and vaccines. Curr HIV/AIDS Rep (2022) 19:5–16. 10.1007/s11904-021-00590-x
103.
YunihastutiEKarjadiTHWidhaniAMahdiHISSundariSHapsariAFet alIncidence and severity prediction score of COVID-19 in people living with HIV (SCOVHIV): experience from the first and second waves of the pandemic in Indonesia. AIDS Res Ther (2022) 19(1):47–8. 10.1186/s12981-022-00472-1
104.
ZhuFCaoYXuSZhouM. Co‐infection of SARS‐CoV‐2 and HIV in a patient in Wuhan city, China. J Med Virol (2020) 92:529–30. 10.1002/jmv.25732
105.
HoffmannCCasadoJLHärterGVizcarraPMorenoACattaneoDet alImmune deficiency is a risk factor for severe COVID‐19 in people living with HIV. HIV Med (2021) 22(5):372–8. 10.1111/hiv.13037
106.
VenturasJZampariniJShaddockEStaceySMurrayLRichardsGAet alComparison of outcomes in HIV-positive and HIV-negative patients with COVID-19. J Infect (2021) 83(2):217–27. 10.1016/j.jinf.2021.05.020
107.
LiuW-DHungCCWangJTTsaiMJKuoPHChaoTLet alEvolution of SARS-CoV-2 neutralizing antibody in an HIV-positive patient with COVID-19. J Formos Med Assoc (2021) 120(12):2186–90. 10.1016/j.jfma.2021.04.010
108.
ZhaoJLiaoXWangHWeiLXingMLiuLet alEarly virus clearance and delayed antibody response in a case of coronavirus disease 2019 (COVID-19) with a history of coinfection with human immunodeficiency virus type 1 and hepatitis C virus. Clin Infect Dis (2020) 71(16):2233–5. 10.1093/cid/ciaa408
109.
SchnittmanSRJungWFitchKVZanniMVMcCallumSLeeJSLet alEffect of host factors and COVID-19 infection on the humoral immune repertoire in treated HIV. JCI insight (2023) 8(5):e166848. 10.1172/jci.insight.166848
110.
DonadeuLTiraboschiJMScévolaSTorijaAMeneghiniMJouveTet alLong-lasting adaptive immune memory specific to SARS-CoV-2 in convalescent coronavirus disease 2019 stable people with HIV. AIDS (2022) 36(10):1373–82. 10.1097/qad.0000000000003276
Summary
Keywords
people living with HIV, immunological response, clinical outcomes, COVID-19, HIV/SARS-CoV-2 coinfection
Citation
Amegashie EA, Asamoah P, Ativi LEA, Adusei-Poku M, Bonney EY, Tagoe EA, Paintsil E, Torpey K and Quaye O (2024) Clinical outcomes and immunological response to SARS-CoV-2 infection among people living with HIV. Exp. Biol. Med. 249:10059. doi: 10.3389/ebm.2024.10059
Received
23 November 2023
Accepted
22 February 2024
Published
02 April 2024
Volume
249 - 2024
Updates
Copyright
© 2024 Amegashie, Asamoah, Ativi, Adusei-Poku, Bonney, Tagoe, Paintsil, Torpey and Quaye.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Osbourne Quaye, oquaye@ug.edu.gh
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.