Abstract
Inflammatory breast cancer (IBC) is a rare and aggressive breast cancer subtype with poor survival. Identifying novel biomarkers is needed to predict survival for this highly progressive form of breast cancer. In this retrospective study, we investigated pan-immune-inflammation value (PIV), a novel immune-inflammation-based biomarker which combined the peripheral blood parameters (lymphocytes, monocytes, neutrophils, and platelets) in a retrospective cohort of 143 IBC patients. Then we explored the difference of PIV levels in IBC and non-IBC cohorts and the relationship between PIV and clinical characteristics in IBC patients. The survival rates of disease-free survival (DFS) and overall survival (OS) in IBC patients were analyzed and univariate and multivariate statistics were used to evaluate the prognostic value. PIV had the most significantly predictive value in IBC patients compared with other peripheral blood parameters. The mean PIV value in IBC patients was significantly higher than non-IBC patients, and the significant difference between the IBC and non-IBC was also observed in subgroups with different clinical stages and pathologic types. Furthermore, PIV performed an extensive systemic immune prognostic factor on both DFS and OS in IBC patients, and PIV was identified an independent prognostic indicator for survival outcome in IBC patients in univariate and multivariate models. Our retrospective study demonstrated the prognostic value of PIV in IBC patients, suggesting the potential application of PIV in IBC treatment outcomes. PIV would also provide some insights into the mechanisms underlying the role of immune and inflammation in IBC development and progression.
Impact Statement
In this work, we identified pan-immune-inflammation value (PIV), a novel immune-inflammation-based biomarker, showed a significantly predictive value in IBC patients, and it's the first time to retrospectively evaluate the predictive value of PIV in IBC patients. We found that PIV had the most significantly predictive value in IBC patients compared with other peripheral blood parameters, and PIV was considered as a favorable independent prognostic indicator in IBC patients. Furthermore, the mean PIV value in IBC patients was significantly higher than non-IBC patients, which might provide some insights into the mechanisms underlying the role of immune and inflammation in IBC development and progression compared with non-IBC. Our retrospective study demonstrated the prognostic value of PIV in IBC patients, suggesting the potential application of PIV in IBC treatment outcomes.
Introduction
Inflammatory breast cancer (IBC) is a rapidly advancing and highly aggressive form of breast cancer [1, 2]. Despite being a relatively rare subtype, IBC accounts for approximately 10% of all breast cancer related deaths [3]. Since no IBC-specific target and treatment strategy have been identified, IBC is mainly treated with the anthracycline/taxane-based chemotherapy with or without anti-HER2 therapy similar to non-IBC [4]. However, patients suffered with IBC usually have shorter survival time and worse prognosis compared with non-IBC cases [5], and there is still controversy about the prognostic evaluation of IBC. Therefore, it is essential to identify novel biomarkers to predict survival, which will contribute to make accurate treatment plans to benefit IBC patients.
Characterized by involvement of skin, the clinicopathological features of IBC are due to the lymphatic obstruction caused by widespread of tumor emboli [6]. There is evidence supporting that the contact between cancer cells and tumor microenvironment (TME) is required for the unique emboli form of IBC [6, 7]. Recent studies have revealed the contributions of the TME to the progressive and invasive behavior of IBC, such as immune evasion and chemotherapy resistance [6, 8, 9]. Among the components in TME, tumor associated macrophages are considered the main immune inflammatory cells of the TME in IBC, which usually polarize to alternatively activated M2 macrophages and act as immunosuppressive cells to induce tumor metastasis [8, 10, 11]. Besides, tumor-infiltrated lymphocytes (TILs) also participate in the controlling and transforming of TME in IBC and play a significant role in initiation and progression of tumor [7]. These individual cells within the TME collectively lead to a unique immune microenvironment of IBC, which provides a novel perspective on investigating the immune features of IBC and evaluating the IBC-associated immune inflammatory markers for forecasting the prognosis and adopting suitable therapeutic strategies.
Over past few years, a number of immune inflammatory biomarkers such as neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), and monocyte to lymphocyte ratio (MLR), are based on blood parameters and easy to evaluate, which have been widely studied and showed their values in predicting the prognosis of breast cancer [12–14]. Given the complexity of the TME in IBC, the pan-immune-inflammation value (PIV) may provide additional information [14]. PIV is a kind of novel score index that combines the counts of these immune inflammatory cells involved may provide more relevant information [15–21]. It was initially used as a tool to predict survival of advanced colorectal cancer [16], and has been gradually discovered its potential value in many other cancer subtypes, especially in breast cancer [15, 17, 18, 22]. However, there is currently no available PIV data could assess the treatment efficacy of IBC. In this article, we conducted a single-center, retrospective assessment, aiming at illustrating prognostic significance of PIV in IBC patients and providing a novel immune biomarker for IBC patients.
Materials and methods
Patient population
Patients clinically diagnosed with IBC in the Harbin Medical University Cancer Hospital during January 2010 to December 2023 were enrolled into this current retrospective, single-center investigation. All cases (n = 143) were clinically defined according to international consensus criteria that the patient exhibited typical clinical features of IBC and fulfilled the pathological T4d diagnosis [1]. The clinic-pathological data of patients, including age, TNM stage, histopathological information (receptor subtype, pathological type, grade, Ki67 and P53 status), body mass index [BMI, as weight (kg) divided by the square of height (m2)] [23] and follow-up details, were collected in accordance with the ethical principles outlined in the Helsinki Declaration regarding research involving human subjects. Neoadjuvant chemotherapy was administered in stage III IBC patients, which was anthracycline-based, incorporating taxanes. Anti-HER2 targeted therapy was combined with the chemotherapy in the cases with HER2-positive status. Subsequently, patients who underwent mastectomy and axillary lymph node dissection were subjected to chemotherapy, endocrine therapy and radiotherapy. However, for stage IV IBC patients, salvage therapies including chemotherapy, anti-HER2 targeted therapy and endocrine therapy were applied according to the molecular subtypes. To evaluate whether the peripheral blood parameters were correlated with the phenotype or with advanced stage in IBC, 168 non-IBC patients were also collected between January 2010 and December 2023 at Harbin Medical University Cancer Hospital. This non-IBC group was randomly sampled to match the IBC cases in the same period and the molecular subtypes when diagnosed. Patients who underwent immunomodulatory treatment or had hematological disease and a history of malignancies were excluded. This study was approved by the Ethics Committee of Harbin Medical University Cancer Hospital, and all patients had written informed consent.
Blood count collection
The pre-treatment peripheral blood data of neutrophil count, lymphocyte count, monocyte count and platelet count were obtained 1 week before any treatment. The absolute counts of neutrophils, lymphocytes, monocytes, and platelets were used to investigate NLR, MLR, and PLR. The PIV was calculated as neutrophil count × platelet count × monocyte count/lymphocyte count [14].
Follow-up
Patients were regularly followed up using a system that combined telephone communication and the follow-up department. The recorded information included patients’ health status, disease progression and date of mortality. DFS (disease-free survival) was calculated as the period (in months) from disease diagnosis until disease recurrence or death. OS (overall survival) was calculated as the time (in months) from disease diagnosis to the date of death due to any cause. The last follow-up date was considered as the survival study endpoint for all patients.
Statistical analysis
ROC curve analysis was performed to determine the cut-off values for PIV, NLR, MLR, and PLR, taking disease recurrence or death as the endpoint of interest. Correlations between high or low PIV groups and clinicopathological features were analyzed using the χ2 or the Fisher’s exact test when appropriate. Two-tailed, unpaired Student’s t tests and one way ANOVA were used to analyze the statistical significance of PIV with clinical parameters between IBC and non-IBC patients. Kaplan-Meier curves were used to visualize survival probabilities over time, the log-rank test was employed to compare survival curves between groups based on the indicator. The Cox regression analyses were conducted to evaluate the influence of clinic-pathological parameters on clinical survival outcomes. All statistical analyses were performed using SPSS 20.0 statistics software (IBM, USA). Statistical significance was defined as p < 0.05 (two-tailed).
Results
Study population
A total of 143 IBC and matched-pair 168 non-IBC patients were enrolled in our study and the clinical-pathological features were described in Table 1. The median age of the IBC patients was 54.5 years (ranging from 28 to 85 years), higher than non-IBC patients with a median age of 51.5 years (ranging from 28 to 85 years). IBC patients were classified into IIIb (69.2%), IIIc (16.1%) and IV (14.7%) stage due to the T4d diagnosis according to the TNM-UICC [24], however in non-IBC patients, 72 patients (42.9%) were in I-IIIa stage. Compared with non-IBC patients, more IBC patients had BMI ≥35 (21.7% vs. 9.5%). The percentage of the molecular subtypes, pathological type, tumor grade and P53 status were similar in IBC and non-IBC cases.
TABLE 1
| Characteristics | Total (n = 311) | IBC (n = 143) | Non-IBC (n = 168) |
|---|---|---|---|
| Age, y | |||
| Median | 53.0 | 54.5 | 51.5 |
| Range | 27–85 | 28–85 | 27–82 |
| Stage | |||
| I-IIIa | 72 (23.2%) | 0 (0.0%) | 72 (42.9%) |
| IIIb | 124 (39.9%) | 99 (69.2%) | 25 (14.9%) |
| IIIc | 58 (18.6%) | 23 (16.1%) | 35 (20.8%) |
| IV | 75 (24.1%) | 21 (14.7%) | 36 (21.4%) |
| Receptor subtype | |||
| HR+/HER2− | 123 (39.5%) | 57 (39.9%) | 66 (39.3%) |
| HER2+ | 119 (38.3%) | 55 (38.5%) | 64 (38.1%) |
| TNBC | 69 (22.2%) | 31 (21.7%) | 38 (22.6%) |
| Pathological type | |||
| Ductal | 263 (84.6%) | 123 (86.0%) | 140 (83.3%) |
| Lobular | 25 (8.0%) | 9 (6.3%) | 16 (9.5%) |
| Mixed/other | 23 (7.4%) | 11 (7.7%) | 12 (7.1%) |
| Grade | |||
| 1 | 9 (2.9%) | 2 (1.4%) | 7 (4.2%) |
| 2 | 177 (56.9%) | 80 (55.9%) | 97 (57.7%) |
| 3 | 125 (40.2%) | 61 (42.7%) | 64 (38.1%) |
| Ki67 status | |||
| Median | 33.0% | 31.0% | 34.0% |
| Range | 0–80% | 0–80% | 0–80% |
| P53 status | |||
| Positive | 134 (43.1%) | 62 (43.4%) | 72 (42.9%) |
| Negative | 177 (56.9%) | 81 (56.6%) | 96 (57.1%) |
| Body mass index (BMI) | |||
| <25 | 45 (14.5%) | 17 (11.9%) | 28 (16.7%) |
| 25- <30 | 128 (41.2%) | 54 (37.8%) | 74 (44.0%) |
| 30- <35 | 91 (29.3%) | 41 (28.7%) | 50 (29.8%) |
| ≥35 | 47 (15.1%) | 31 (21.7%) | 16 (9.5%) |
Baseline clinico-pathological parameters of patients in IBC cohorts and non-IBC control cohorts.
P-values were shown in bold values if they had statistical significance.
Prognostic values of blood-based biomarkers in IBC
To investigate the predictive value of immune biomarkers in IBC, ROC curve analysis was conducted and the area under curves (AUC) and cut-off values of PIV and other related biomarkers (MLR, PLR, and NLR) were shown in Figure 1; Table 2. Compared with other markers, PIV showed a better prognostic significance of IBC (AUC = 0.725, P < 0.001). Although PLR (AUC = 0.634, P = 0.014) and NLR (AUC = 0.622, P = 0.025) also had predictive values in IBC, the sensitivity and specificity of them were lower than PIV. Therefore, we considered PIV as the most significantly predictive value in IBC patients, and the cut-off value for the PIV was determined as 284.66.
FIGURE 1
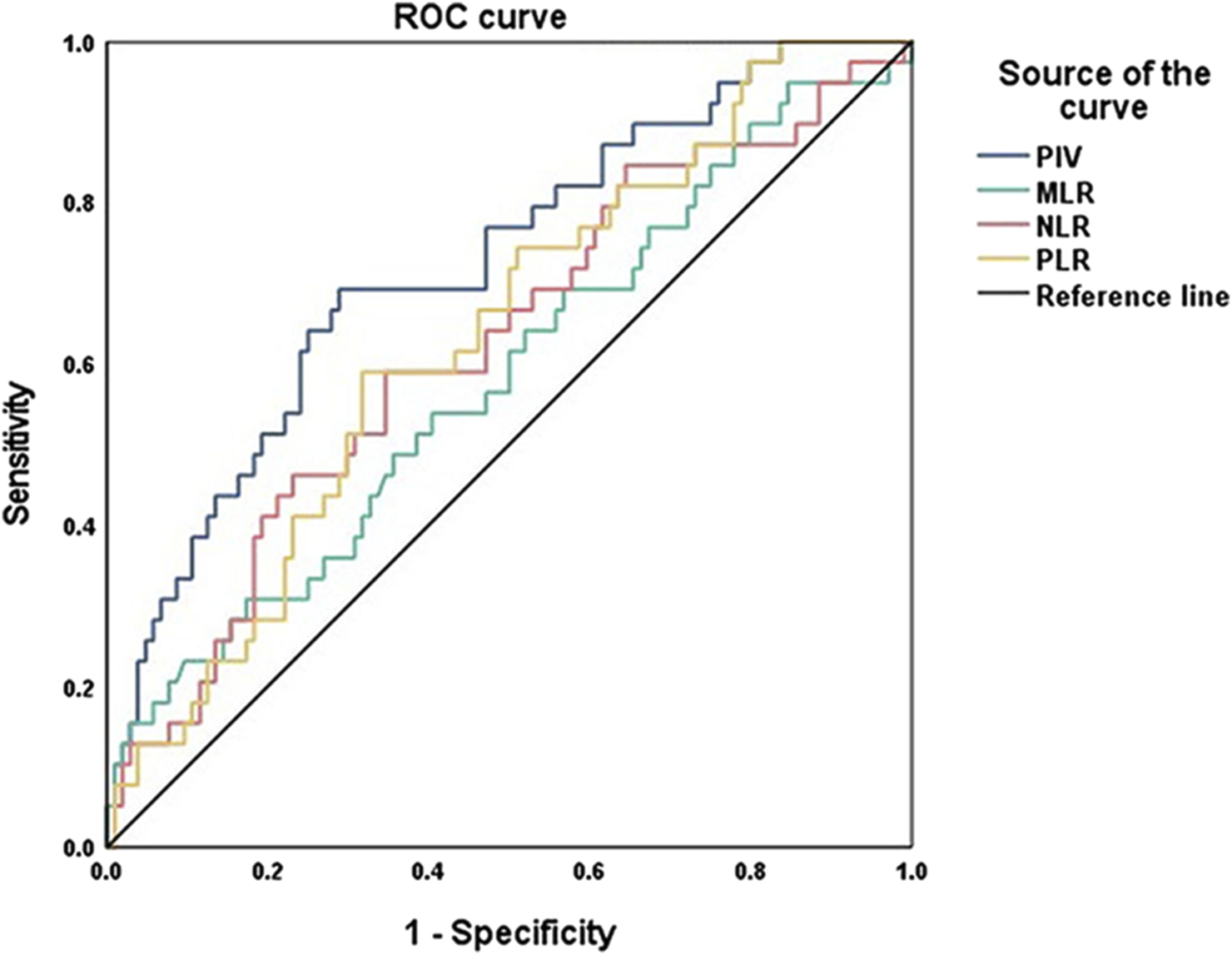
ROC curve analysis of PIV, MLR, PLR and NLR values in IBC patients.
TABLE 2
| Parameters | AUC | 95% CI | Cut-off | Sensitivity (%) | Specificity (%) | P-value |
|---|---|---|---|---|---|---|
| PIV | 0.725 | 0.631–0.818 | 284.66 | 69.2 | 71.2 | <0.001 |
| MLR | 0.583 | 0.476–0.690 | 0.25 | 53.8 | 59.6 | 0.127 |
| PLR | 0.634 | 0.535–0.732 | 162.14 | 59.0 | 68.3 | 0.014 |
| NLR | 0.622 | 0.518–0.727 | 2.50 | 59.0 | 65.4 | 0.025 |
Predictive values of PIV, MLR, PLR and NLR on IBC survival.
Abbreviations: AUC, the area under the curve; CI, confidence interval; PIV, pan-immune-inflammation-value; MLR, monocyte-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; NLR, neutrophil-to-lymphocyte ratio.
P-values were shown in bold values if they had statistical significance.
PIV distinguished IBC from non-IBC cohorts
Since PIV was a comprehensive immune-associated biomarker, we explored whether IBC had different PIV levels compared to non-IBC cohorts. Overall, the mean PIV values of IBC were significantly higher than that in non-IBC patients (Figure 2A). Furthermore, patients were categorized by different clinical stages or receptor subtypes. The results showed that stage IV tumors had higher PIV values in both IBC and non-IBC groups, and the mean PIV values in IBC were also higher than non-IBC patients whether their tumors were stage IIIb, IIIc and IV (Figure 2B). According to receptor subtypes, the patients were classified into HR (hormone receptor)+HER2-, HER2+ and TNBC (triple negative breast cancer) subtypes, and we found that PIV was significantly elevated in IBC cases in all pathological types (Figure 2C). These results indicated that the PIV in IBC patients was a characteristic biomarker, which could distinguish IBC from non-IBC cohorts.
FIGURE 2
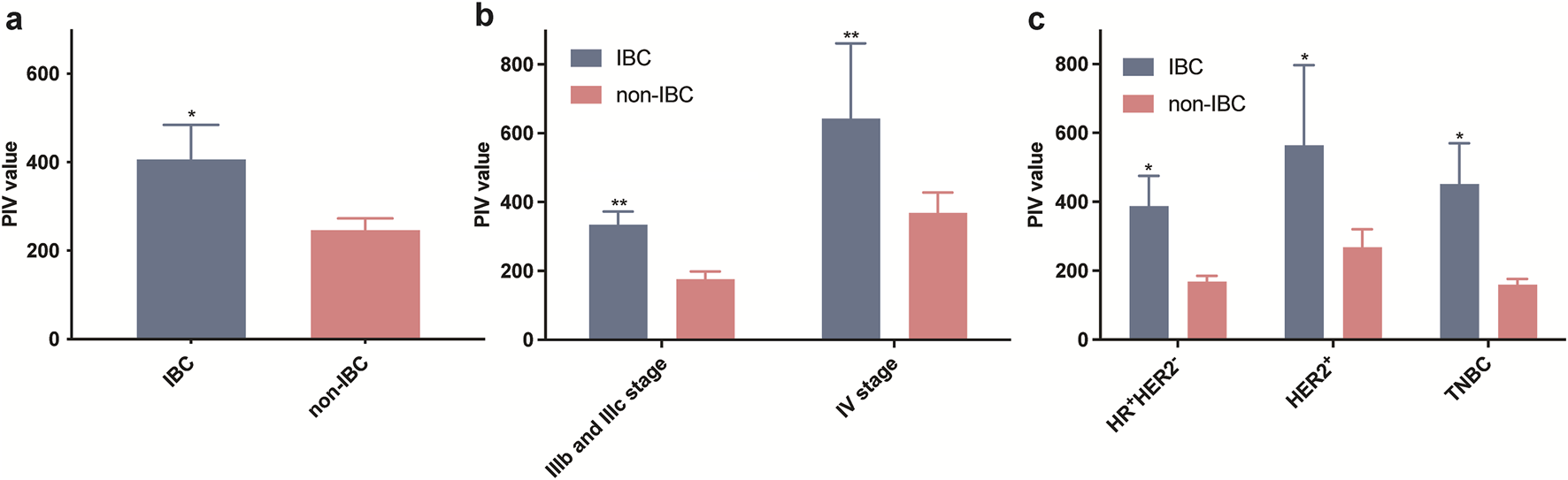
Comparison of PIV values in all IBC and non-IBC patients (a) as well as in different stages (b) and receptor subtypes (c) among IBC and non-IBC patients.
IBC patient characteristics according to PIV value
To further investigate the role of PIV in IBC, the IBC patients were classified into high or low PIV groups according to the calculated PIV cut-off value of 284.66. A comprehensive overview of clinical-pathological features according to PIV are summarized in Table 3. In our study population, 58 (40.6%) patients presented a high PIV, which displayed a higher incidence of lobular pathological type (P = 0.047) and advanced tumor stage (P = 0.040), suggesting a more aggressive metastasis profile in high PIV group. However, there were no significant differences in age, receptor subtype, grade, ki-67 status, p53 status, and BMI.
TABLE 3
| Characteristics | Total (n = 143) | PIV < 284.66 (n = 85) | PIV ≥ 284.66 (n = 58) | P-value |
|---|---|---|---|---|
| Age, y | ||||
| ≤50 | 51 (35.7%) | 29 (34.1%) | 22 (37.9%) | 0.640 |
| >50 | 92 (64.3%) | 56 (65.9%) | 36 (62.1%) | |
| Stage | ||||
| IIIb | 99 (69.2%) | 60 (70.6%) | 39 (67.2%) | 0.040 |
| IIIc | 23 (16.1%) | 9 (10.6%) | 14 (24.1%) | |
| IV | 21 (14.7%) | 16 (18.8%) | 5 (8.6%) | |
| Receptor subtype | ||||
| HR+/HER2− | 57 (39.9%) | 35 (41.2%) | 22 (37.9%) | 0.839 |
| HER2+ | 55 (38.5%) | 31 (36.5%) | 24 (41.4%) | |
| TNBC | 31 (21.7%) | 19 (22.4%) | 12 (20.7%) | |
| Pathological type | ||||
| Ductal | 123 (86.0%) | 75 (88.2%) | 48 (82.8%) | 0.047 |
| Lobular | 9 (6.3%) | 2 (2.4%) | 7 (12.1%) | |
| Mixed/other | 11 (7.7%) | 8 (9.4%) | 3 (5.2%) | |
| Grade | ||||
| 1 | 2 (1.4%) | 2 (2.4%) | 0 (0.0%) | 0.476 |
| 2 | 80 (55.9%) | 48 (56.5%) | 32 (55.2%) | |
| 3 | 61 (42.7%) | 35 (41.2%) | 26 (44.8%) | |
| Ki67 status | ||||
| <20% | 49 (34.3%) | 30 (35.3%) | 19 (32.8%) | 0.754 |
| ≥20% | 94 (65.7%) | 55 (64.7%) | 39 (67.2%) | |
| P53 status | ||||
| Positive | 62 (43.4%) | 35 (41.2%) | 27 (46.6%) | 0.524 |
| Negative | 81(56.6%) | 50 (58.8%) | 31 (53.4%) | |
| Body mass index (BMI) | ||||
| <25 | 17 (11.9%) | 12 (14.1%) | 5 (8.6%) | 0.282 |
| 25- <30 | 54 (37.8%) | 34 (40.0%) | 20 (34.5%) | |
| 30- <35 | 41 (28.7%) | 25 (29.4%) | 16 (27.6%) | |
| ≥35 | 31 (21.7%) | 14 (16.5%) | 17 (29.3%) | |
PIV distribution according to clinico-pathological characteristics in IBC.
P-values were shown in bold values if they had statistical significance.
Survival outcomes according to PIV value in IBC patients
Kaplan-Meier plots illustrated the relationship of PIV value with DFS and OS of IBC patients, and the results showed that the survival outcomes of IBC patients in the high PIV group were markedly worse than that in the low PIV group in both DFS and OS rates (Figure 3A). Furthermore, to better comprehend the impact of PIV on the prognosis of IBC patients in clinical stages and pathological types under various conditions, we conducted subgroup analyses and observed that similar results were observed in different clinical stages (non-IV and IV stage) of IBC patients (Figures 3B,C). However, in terms of different pathological subtypes, no significant difference in DFS or OS rates was observed in HR+HER2- IBC patients based on high or low PIV values (Figure 4A), but HER2+ and TNBC IBC patients in high PIV group had statistically significantly worse survival rates (Figures 4B,C). As a result of univariate and multivariate analyses, PIV all appeared as an independent predictor in DFS and OS outcomes (Table 4). Besides, in multivariate analysis comprising the variables, MLR caused a statistically significant difference in DFS and OS survival outcomes, and P53 made a difference in DFS survival (Table 4). These results indicated that PIV was an independent value to predict survival in IBC patients.
FIGURE 3
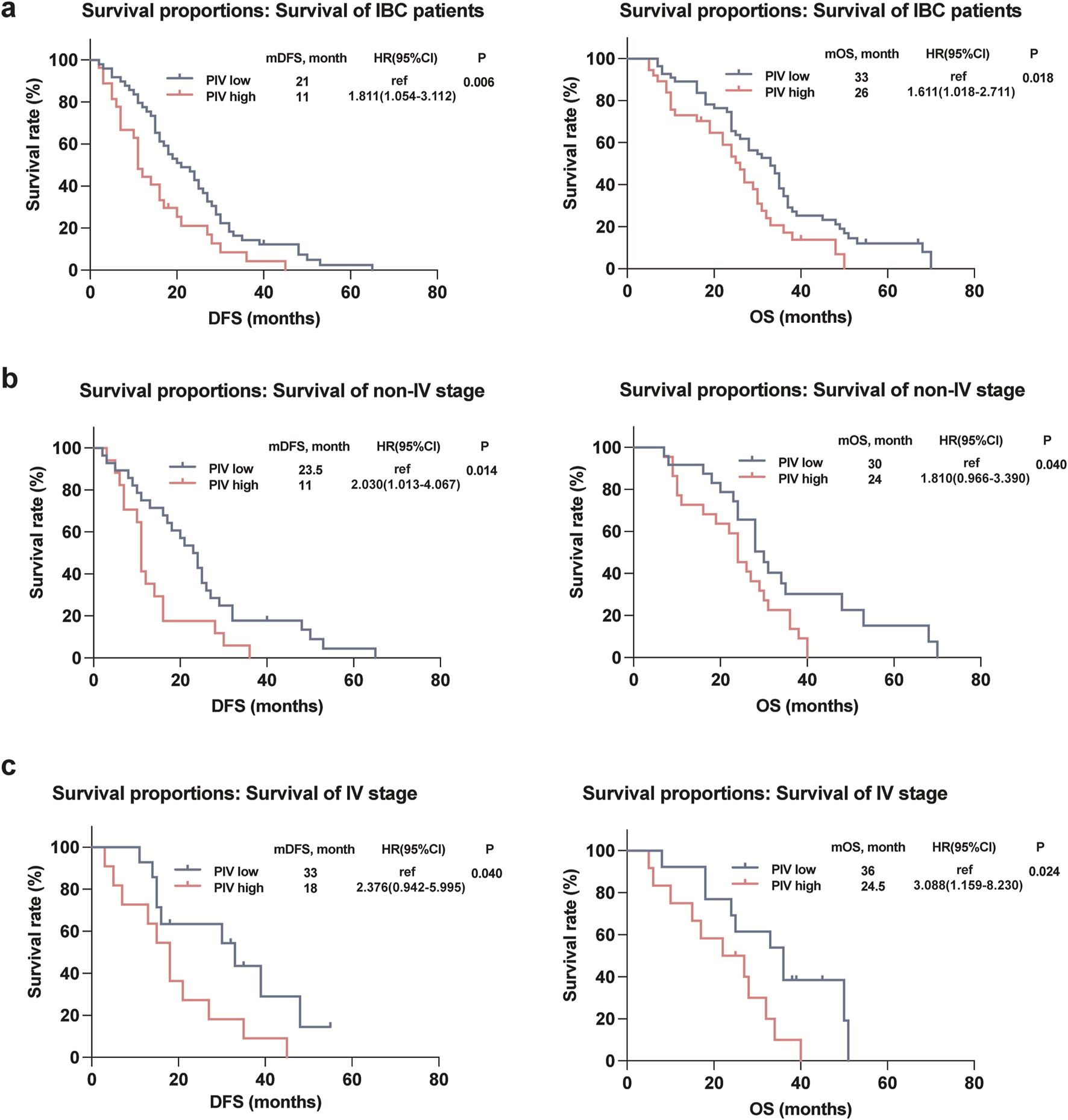
Kaplan-Meier curves for DFS and OS in the IBC patients (a) as well as the IBC patients with different stages (b, c) according to PIV.
FIGURE 4
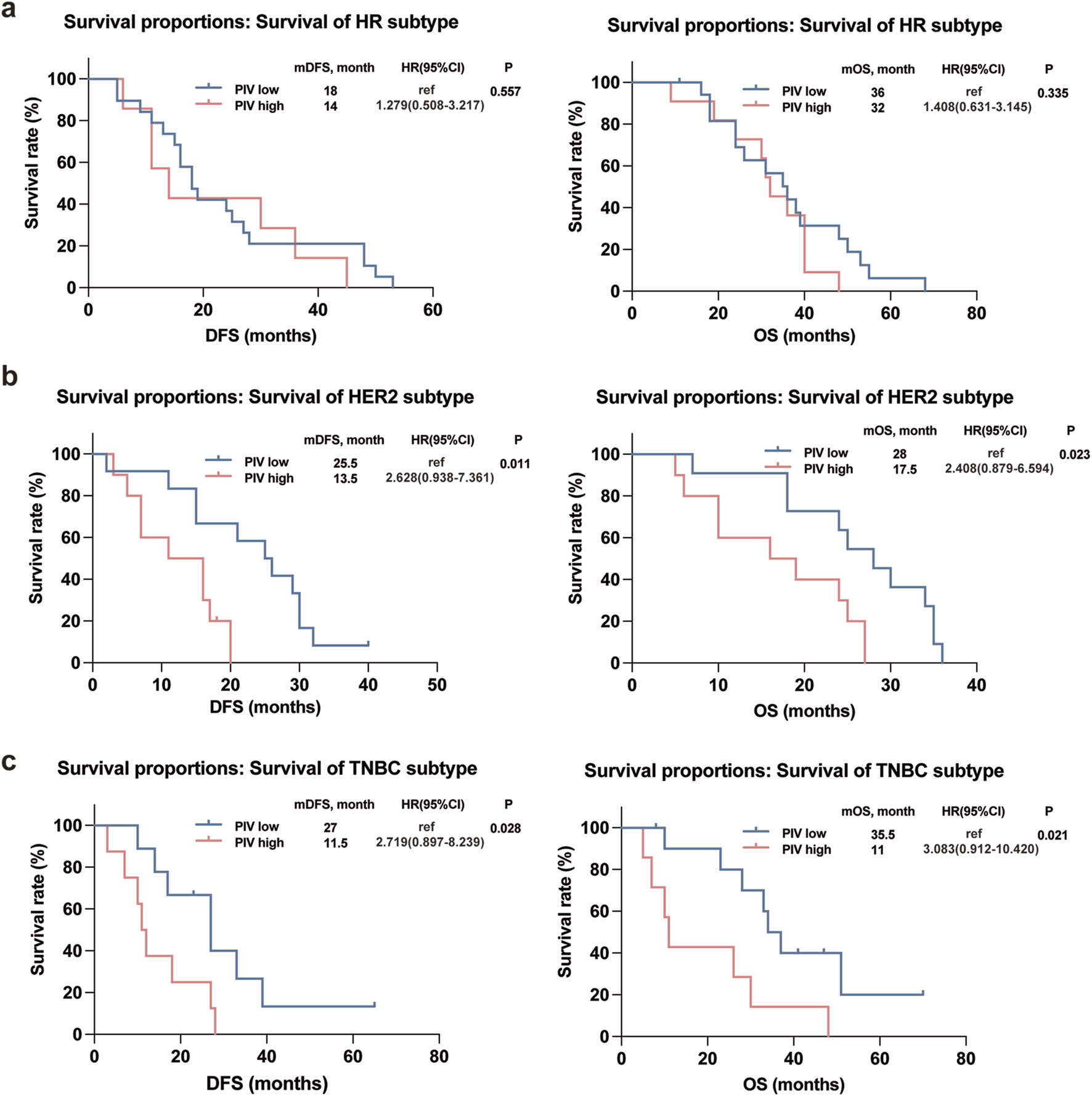
Kaplan-Meier curves for DFS and OS in the IBC patients with different subtypes according to PIV: HR subtype (a); HER2 subtype (b); TNBC subtype (c).
TABLE 4
| Covariate | DFS | OS | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | |||||
| HR (95%CI) | P-value | HR (95%CI) | P-value | HR (95%CI) | P-value | HR (95%CI) | P-value | |
| Age, y (≤50 vs. >50) | 0.971 (0.601–1.569) | 0.905 | 0.989 (0.575–1.701) | 0.969 | 0.972 (0.612–1.545) | 0.906 | 1.118 (0.665–1.877) | 0.674 |
| Stage (IIIb and IIIc vs. IV) | 0.883 (0.660–1.182) | 0.404 | 0.795 (0.584–1.081) | 0.143 | 0.931 (0.709–1.222) | 0.607 | 0.876 (0.661–1.162) | 0.359 |
| Receptor subtype (HR+/HER2- vs. HER2+ vs. TNBC) | 1.052 (0.796–1.391) | 0.722 | 1.060 (0.754–1.490) | 0.736 | 1.115 (0.850–1.462) | 0.433 | 1.268 (0.918–1.751) | 0.149 |
| Pathological type (ductal vs. lobular vs. mixed/other) | 1.059 (0.689–1.626) | 0.795 | 0.848 (0.503–1.432) | 0.538 | 1.073 (0.746–1.543) | 0.705 | 0.946 (0.620–1.444) | 0.797 |
| Grade (1 vs. 2 vs. 3) | 1.062 (0.847–1.332) | 0.603 | 1.118 (0.863–1.447) | 0.398 | 1.054 (0.845–1.314) | 0.641 | 1.166 (0.911–1.491) | 0.222 |
| Ki67 status (<20% vs. ≥20%) | 1.139 (0.710–1.827) | 0.588 | 1.056 (0.606–1.840) | 0.848 | 1.057 (0.674–1.654) | 0.809 | 0.828 (0.490–1.397) | 0.479 |
| P53 status (positive vs. negative) | 1.512 (0.948–2.412) | 0.082 | 1.965 (1.113–3.467) | 0.020 | 1.142 (0.734–1.778) | 0.556 | 1.227 (0.734–2.050) | 0.435 |
| BMI (<25 and 25- <30 vs. 30- <35 and ≥35 | 1.037 (0.647–1.663) | 0.880 | 1.233 (0.698–2.177) | 0.470 | 1.312 (0.833–2.066) | 0.241 | 1.382 (0.837–2.281) | 0.206 |
| PIV (<284.66 vs. ≥284.66) | 1.637 (1.032–2.596) | 0.036 | 1.928 (1.098–3.387) | 0.022 | 1.580 (1.017–2.455) | 0.042 | 1.991 (1.182–3.353) | 0.010 |
| MLR (<0.25 vs. ≥0.25) | 0.781 (0.494–1.236) | 0.292 | 0.401 (0.211–0.762) | 0.005 | 0.817 (0.525–1.273) | 0.372 | 0.405 (0.219–0.748) | 0.004 |
| NLR (<162.14 vs. ≥162.14) | 1.161 (0.738–1.827) | 0.519 | 1.050 (0.504–2.187) | 0.897 | 1.298 (0.833–2.023) | 0.249 | 1.640 (0.885–3.037) | 0.116 |
| PLR (<2.50 vs. ≥2.50) | 1.185 (0.747–1.882) | 0.471 | 1.514 (0.772–2.967) | 0.227 | 1.183 (0.755–1.853) | 0.464 | 1.400 (0.770–2.547) | 0.270 |
Univariate and Multivariate analysis of prognostic factors for survival in IBC patients.
Discussion
In this study, we investigated PIV, a novel immune-inflammation-based biomarker which contained the majority of immune inflammatory cell components in peripheral blood (lymphocytes, monocytes, neutrophils, and platelets), in a retrospective cohort of 143 IBC patients. First, we demonstrated that PIV had the most significantly predictive value in IBC patients. The mean PIV value in IBC patients was significantly higher compared to non-IBC patients, and the significant difference between the IBC and non-IBC was also observed in subgroups with different pathologic types and different clinical stages. Our results were in line with the well-documented perception that there was a distinct immune features and characteristic inflammation markers for IBC. Furthermore, PIV performed an extensive and favorable systemic immune prognostic factor on both DFS and OS in IBC patients, and PIV was identified an independent prognostic indicator for survival outcome in IBC patients with univariate and multivariate analyses. To our knowledge, these are novel results to estimate the prognostic value of PIV in IBC cohort.
Peripheral immune components or system is the fundamental for the orchestration and maintenance of the tumor-perturbed immune system, and also provide effective biomarkers for the diagnosis and prognosis of cancer and response to therapy [14, 25, 26]. There are studies that focusing on the immune profile of IBC, and the heterogeneous immune landscape of IBC was pointed out, which has improved our understanding of the immune characteristics of IBC [27]. In our study, PIV value in IBC patients was significantly higher compared with non-IBC patients, not only observed in different pathologic types, but also in different clinical stages, suggesting a potential distinct immune feature in IBC, that specific immune cell types of IBC may play a role in the progression and response of therapy.
Previous studies have usually used single immune component counting or the ratio of two to reflect peripheral immune system status in the prognostic modelling of IBC [12]. Lymphocytes are pivotal and multifaceted components in the anti-tumor immune response [28]. Recent research has shown that heterogeneous immune profiles in patients with IBC could impact on cancer immunity and be associated with clinical response [27]. It has been previously described that the most remarkable feature of peripheral blood in IBC was extreme lymphopenia that was highly correlated with the IBC disease itself rather than with treatment, and showed significant reduction in most subpopulations of lymphocytes [7]. As equivalently matched opponents with lymphocytes, circulating monocytes are another essential phenotype of myeloid immune cells, which are trafficked to the TME, divided into different subpopulations, and eventually contribute to local immunosuppression [29]. Previous studies have found that monocytes counting in the IBC peripheral blood was more than in non-IBC patients [11].
In contrast to the predominant contributions of the above-mentioned types, there are a couple of other components in peripheral blood, such as neutrophils and platelets [30, 31]. Neutrophils and platelets are thought to be frequently replenished from common myeloid progenitor (CMP) shared with monocytes [14]. Despite being great enrichment in peripheral immune system and accumulating in a wide range of cancer, neutrophils and platelets have been implicated to emerge as not isolated performers, but rather the key mediators and crosstalk in the cancer immune systems [30]. Previous studies demonstrated that integration index (neutrophil to lymphocyte ratio or platelets to lymphocyte ratio) was associated with tumor burden and clinical parameters, such as the optimal candidate biomarker for IBC patients [12].
Compared to previous indicators (counting or ratio) with fragmented, partial information, PIV has served as comprehensive, integrated peripheral immune biomarker incorporating the globally immune components (lymphocytes, monocytes, neutrophils, and platelets) [15–17, 21, 32]. PIV initially emerged as the prognostic indicator for patients with metastatic colorectal cancer [16], and the similar prognostic significance of PIV has been well established in various malignant tumors and immune and inflammation-related disorders [19–21]. In this study, the result showed that PIV could provide more effective value in IBC compared with other prognostic markers, MLR, NLR, and PLR. This finding was similar to the results from retrospective studies in the literature of breast cancer not only in advanced stage but also in the early-stage breast cancer patients [15, 17, 18, 22, 33]. When PIV value was applied for stratification of IBC patients, it became evident that higher PIV was associated with a more unfavorable prognosis. Our study findings indicated a pronounced inverse relationship between elevated PIV levels and both DFS and OS in patients. Moreover, based on both univariate and multivariate COX regression analyses, we observed that PIV was an independent value to predict survival in IBC patients.
The present study had some limitations. First, it was a retrospective study by single-center design, and IBC showed a relatively rare incidence, with a limited number of IBC patients, which might lead to unanticipated biases, such as selection, information and confounding biases. Second, although we excluded the patients who received immunomodulatory treatment, there existed other conditions influencing the blood-based biomarkers. Third, our study did not incorporate tumor genomic or immune microenvironmental data, which may influence IBC outcomes. Besides, although we have incorporated the metabolic indicator BMI in our study, other metabolic comorbidities such as diabetes and metabolic syndrome, which may influence systemic inflammation. Finally, more detailed analysis, such as the interplay between molecular subtype, lymphovascular invasion or host metabolic status with tumor-associated inflammation in IBC remains unexplored, which are warranting future prospective studies. Although we have employed multiple strategies to minimize the biases, further validation through randomized multicenter studies is needed to be conducted to confirm observation in this study.
Conclusion
In summary, this translational retrospective study demonstrated the prognostic significance of PIV in IBC patients, which outperformed other blood-based immune markers, suggesting its potential application in predicting IBC treatment outcomes. Higher PIV value in IBC patients compared with subtype-matched cohort would also provide some insights into the mechanisms underlying the role of immune and inflammation in IBC development and progression.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Harbin Medical University Cancer Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by YH, JL, MW, XW, and JL. The first draft of the manuscript was written by XN and HJ and all authors commented on previous versions of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Haiyan Foundation of Harbin Medical University Cancer Hospital under Grant No. JJZD2024-18, Harbin Medical University Cancer Hospital Top Young Talent Project under Grant No. BJQN2021-03, and the National Science Foundation of Heilongjiang Province under grant Nos PL2024H179 and LH2023H085.
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
References
1.
ManaiMFinettiPMejriNAthimniSBirnbaumDBertucciFet alInflammatory breast cancer in 210 patients: a retrospective study on epidemiological, anatomo-clinical features and therapeutic results. Mol Clin Oncol (2019) 10:223–30. 10.3892/mco.2018.1773
2.
van UdenDJPvan LaarhovenHWMWestenbergAHde WiltJHWBlanken-PeetersCFJM. Inflammatory breast cancer: an overview. Crit Rev Oncology/Hematology (2015) 93:116–26. 10.1016/j.critrevonc.2014.09.003
3.
HesterRHHortobagyiGNLimB. Inflammatory breast cancer: early recognition and diagnosis is critical. Am J Obstet Gynecol (2021) 225:392–6. 10.1016/j.ajog.2021.04.217
4.
DawoodSMerajverSDViensPVermeulenPBSwainSMBuchholzTAet alInternational expert panel on inflammatory breast cancer: consensus statement for standardized diagnosis and treatment. Ann Oncol (2011) 22:515–23. 10.1093/annonc/mdq345
5.
HanceKWAndersonWFDevesaSSYoungHALevinePH. Trends in inflammatory breast carcinoma incidence and survival: the surveillance, epidemiology, and end results program at the National Cancer Institute. JNCI: J Natl Cancer Inst (2005) 97:966–75. 10.1093/jnci/dji172
6.
LimBWoodwardWAWangXReubenJMUenoNT. Inflammatory breast cancer biology: the tumour microenvironment is key. Nat Rev Cancer (2018) 18:485–99. 10.1038/s41568-018-0010-y
7.
FernandezSVMacFarlaneAWJillabMArisiMFYearleyJAnnamalaiLet alImmune phenotype of patients with stage IV metastatic inflammatory breast cancer. Breast Cancer Res (2020) 22:134–16. 10.1186/s13058-020-01371-x
8.
QuailDFJoyceJA. Microenvironmental regulation of tumor progression and metastasis. Nat Med (2013) 19:1423–37. 10.1038/nm.3394
9.
HuangACaoSTangL. The tumor microenvironment and inflammatory breast cancer. J Cancer (2017) 8:1884–91. 10.7150/jca.17595
10.
SuSLiuQChenJChenJChenFHeCet alA positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell (2014) 25:605–20. 10.1016/j.ccr.2014.03.021
11.
Di BonitoMCantileMBottiG. Pathological and molecular characteristics of inflammatory breast cancer. Transl Cancer Res (2019) 8:S449–S456. 10.21037/tcr.2019.03.24
12.
Van BerckelaerCVan GeytMLindersSRypensCTrinhXBTjalmaWAAet alA high neutrophil-lymphocyte ratio and platelet-lymphocyte ratio are associated with a worse outcome in inflammatory breast cancer. The Breast (2020) 53:212–20. 10.1016/j.breast.2020.08.006
13.
Pivatto JúniorFSantosÂBSEnglertEFMazzuttiGCostaGOMSaffiMALet alMonocyte-to-lymphocyte ratio as predictor of cancer therapy-related cardiotoxicity in patients with breast cancer: a pilot cohort study. Breast Cancer Res Treat (2023) 200:355–62. 10.1007/s10549-023-06979-z
14.
XieJGuoZZhuYMaMJiaG. Peripheral blood inflammatory indexes in breast cancer: a review. Medicine (2023) 102:E36315. 10.1097/md.0000000000036315
15.
QiXQiaoBSongTHuangDZhangHLiuYet alClinical utility of the pan-immune-inflammation value in breast cancer patients. Front Oncol (2023) 13:1223786. 10.3389/FONC.2023.1223786
16.
FucàGGuariniVAntoniottiCMoranoFMorettoRCoralloSet alThe Pan-Immune-Inflammation Value is a new prognostic biomarker in metastatic colorectal cancer: results from a pooled-analysis of the Valentino and TRIBE first-line trials. Br J Cancer (2020) 123:403–9. 10.1038/s41416-020-0894-7
17.
LinFZhangLPXieSYHuangHYChenXYJiangTCet alPan-immune-inflammation value: a new prognostic index in operative breast cancer. Front Oncol (2022) 12:830138. 10.3389/FONC.2022.830138
18.
SahinTKAkyildizADoganOTKavgaciGGuvenDCAksoyS. Prognostic significance of pan-immune-inflammation value in patients with HER2-positive metastatic breast cancer treated with trastuzumab emtansine. Pharmaceuticals (Basel) (2024) 17:824. 10.3390/ph17070824
19.
GambichlerTSaidSAbu RachedNScheelCHSusokLStranzenbachRet alPan-immune-inflammation value independently predicts disease recurrence in patients with Merkel cell carcinoma. J Cancer Res Clin Oncol (2022) 148:3183–9. 10.1007/s00432-022-03929-y
20.
FuMZhangXShenFMaJLiZ. Prognostic value of peripheral blood neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, pan-immune-inflammation value and systemic immune-inflammation index for the efficacy of immunotherapy in patients with advanced gastric cancer. Immunotherapy (2024) 16:551–63. 10.2217/imt-2024-0031
21.
TopkanESelekUKucukAPehlivanB. Low pre-ChemoradiotherapyPan-immune-inflammation value (PIV) measures predict better survival outcomes in locally advanced pancreatic adenocarcinomas. J Inflamm Res (2022) 15:5413–23. 10.2147/jir.s385328
22.
ProvenzanoLLobefaroRLigorioFZattarinEZambelliLSposettiCet alThe pan-immune-inflammation value is associated with clinical outcomes in patients with advanced TNBC treated with first-line, platinum-based chemotherapy: an institutional retrospective analysis. Ther Adv Med Oncol (2023) 15:17588359231165978. 10.1177/17588359231165978
23.
SchairerCLaurentCAMoyLMGierachGLCaporasoNEPfeifferRMet alObesity and related conditions and risk of inflammatory breast cancer: a nested case–control study. Breast Cancer Res Treat (2020) 183:467–78. 10.1007/s10549-020-05785-1
24.
FouadTMBarreraAMGReubenJMLucciAWoodwardWAStauderMCet alInflammatory breast cancer: a proposed conceptual shift in the UICC-AJCC TNM staging system. The Lancet Oncol (2017) 18:e228–e232. 10.1016/s1470-2045(17)30192-4
25.
YangRChangQMengXGaoNWangW. Prognostic value of Systemic immune-inflammation index in cancer: a meta-analysis. J Cancer (2018) 9:3295–302. 10.7150/jca.25691
26.
GianniCPalleschiMSchepisiGCasadeiCBleveSMerloniFet alCirculating inflammatory cells in patients with metastatic breast cancer: implications for treatment. Front Oncol (2022) 12:882896. 10.3389/fonc.2022.882896
27.
BertucciFBoudinLFinettiPVan BerckelaerCVan DamPDirixLet alImmune landscape of inflammatory breast cancer suggests vulnerability to immune checkpoint inhibitors. Oncoimmunology (2021) 10:1929724. 10.1080/2162402X.2021.1929724
28.
SharmaPHu-LieskovanSWargoJARibasA. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell (2017) 168:707–23. 10.1016/j.cell.2017.01.017
29.
MegoMGaoHCohenENAnfossiSGiordanoASandaTet alCirculating tumor cells (CTC) are associated with defects in adaptive immunity in patients with inflammatory breast cancer. J Cancer (2016) 7:1095–104. 10.7150/jca.13098
30.
StoiberDAssingerA. Platelet-leukocyte interplay in cancer development and progression. Cells (2020) 9:855. 10.3390/CELLS9040855
31.
SagivJYMichaeliJAssiSMishalianIKisosHLevyLet alPhenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep (2015) 10:562–73. 10.1016/j.celrep.2014.12.039
32.
GuvenDCSahinTKErulEKilickapSGambichlerTAksoyS. The association between the Pan‐Immune‐Inflammation value and cancer prognosis: a systematic review and meta‐analysis. Cancers (2022) 14:2675. 10.3390/cancers14112675
33.
ŞahinABCubukcuEOcakBDeligonulAOyucu OrhanSTolunaySet alLow pan-immune-inflammation-value predicts better chemotherapy response and survival in breast cancer patients treated with neoadjuvant chemotherapy. Sci Rep (2021) 11:14662. 10.1038/s41598-021-94184-7
Summary
Keywords
inflammatory breast cancer, pan-immune-inflammation value, prognosis, biomarker, immune
Citation
Hu Y, Li J, Wang M, Wang X, Li J, Ji H and Niu X (2025) Pan-immune-inflammation value predicts survival in inflammatory breast cancer patients. Exp. Biol. Med. 250:10493. doi: 10.3389/ebm.2025.10493
Received
16 January 2025
Accepted
14 April 2025
Published
01 May 2025
Volume
250 - 2025
Updates
Copyright
© 2025 Hu, Li, Wang, Wang, Li, Ji and Niu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingjian Niu, niuxingjian@hrbmu.edu.cn
‡These authors have contributed equally to this work
ORCID: Xingjian Niu, orcid.org/0000-0001-8210-0168
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.