Abstract
The relationship between drinking and sarcopenia remains controversial. The aim of the present study was to investigate the association of alcohol drinking with sarcopenia in the older adults. A prospective study with 5244 Chinese community-dwelling older adults aged ≥65 years was performed. Sarcopenia was assessed by appendicular skeletal muscle mass index, grip strength, and gait speed. A quantitative questionnaire was used to obtain the information of alcohol drinking. After 4-year follow-up, our study showed that drinkers had lower incidence of sarcopenia than those non-drinkers (19.4% vs. 30.4%, P < 0.001 in males and 9.5% vs. 20.4%, P = 0.004 in females, respectively). Moreover, male drinkers had higher levels of muscle mass [median (IQR): 7.3 (6.7–7.9) kg/m2 vs. 7.1 (6.5–7.7) kg/m2, P < 0.001] grip strength [median (IQR): 31.1 (26.5–35.0) kg vs. 29.6 (24.8–38.8) kg, P < 0.001], and gait speed [median (IQR): 1.08 (0.98–1.17) m/s vs. 1.05 (0.94–1.15) m/s, P < 0.001] than those non-drinkers, while female drinkers had higher gait speed [median (IQR): 1.02 (0.94–1.11) m/s vs. 0.99 (0.89–1.09) m/s, P = 0.031] than those non-drinkers. Multivariate logistic regression showed that in older adults younger than 85 years, both interim drinking (RR = 0.60; 95%CI = 0.39–0.93; P = 0.021 for males; RR = 0.36; 95%CI = 0.13–0.90; P = 0.035 for females) and daily drinking (RR = 0.78; 95%CI = 0.61–0.99; P = 0.045 for males; RR = 0.34; 95%CI = 0.12–0.96; P = 0.041 for females) were correlated with decreased risk of sarcopenia even after adjustment for confounding factors. However, our dose-response analysis did not show any significant relationship between daily alcohol intake and the risk of sarcopenia as well as the components of sarcopenia. In conclusion, our results indicated that alcohol drinking may not be a risk factor for sarcopenia in the older adults. Further research will help to understand the underlying mechanism of the observed causal relationship.
Impact statement
Although the possible role of alcohol consumption in sarcopenia has attracted increasing attention, the results of current studies remain controversial. Our prospective study provides a novel information that alcohol consumption may not be a risk factor for sarcopenia in a community-dwelling population of Chinese older adults. Specifically, alcohol consumption might have a potential protective effect against the risk of sarcopenia in older adults younger than 85 years old and those who were not underweight (BMI≥18.5 kg/m2). However, heavy drinking has significant social burden in addition to human health and therefore is not recommended. Advices regarding the health effects of alcohol drinking on sarcopenia need to be further individualized according to the specific medical status.
Introduction
Sarcopenia has been identified as a novel geriatric disorder characterized by loss of muscle mass and muscle strength compromising with age [1]. The prevalence of sarcopenia in the older adult ranges from 6% to 12% worldwide [2], and 10.6%–38.8% in China [3–6]. Emerging evidence have linked sarcopenia with a variety of adverse outcomes including frailty, falls, fracture, morbidity and mortality, leading sarcopenia becomes a heavy burden and hotspot in the society of geriatrics [7].
Lifestyle is closely related to sarcopenia, among which drinking is one of the most modifiable behaviors [8]. Drinking is a traditional cultural behavior in China, with the drinking rate of 36.5% and 8.1% in aged male and female, respectively [9]. Although the possible role of alcohol consumption in sarcopenia has attracted increasing attention, the results of current studies remain controversial. Three studies based on Asian population suggested that alcohol drinking might be risk factor for sarcopenia [10–12], whereas another study did not found any correlation between alcohol consumption and sarcopenia [13]. Intriguingly, meta-analysis even indicated that alcohol intake might have protective effect on sarcopenia, especially in males [14, 15], while another recently published meta-analysis reported negative result [16]. The inconsistent results may attribute to the difference of diagnostic criteria for sarcopenia as well as the lack of the specificity of drinking information, such as drinking frequency and consumed alcohol volume. Therefore, the present study aimed to investigate the association of alcohol consumption with sarcopenia in a larger sample size with Chinese community-dwelling older adults.
Materials and methods
Study participants
This prospective study was based on participants from the National Basic Public Health Project in Yuetang Community Medical Center in Yangzhou, Jiangsu Province, China in 2020. Participants completed the general information questionnaire (including alcohol intake), physical examinations (including the collection of blood samples), and anthropometry information. A total of 5976 older adults aged ≥65 years were recruited. The exclusion criteria included: (1) unable to accomplish the specified actions (n = 173); (2) had a history of malignant tumors, dementia, mental disorders, severe cardiopulmonary dysfunction (New York Heart Association class III-IV) (n = 537); (3) With missing drinking information (n = 22). Finally, 5244 older adults were included in the follow-up study. This study was performed in accordance with the principles stated in the Declaration of Helsinki [17] and approved by the Ethics Committee of Sir Run Run Hospital, Nanjing Medical University (approval No. 2019-SR-S041). Written informed consent was obtained from each participant.
Assessment of alcohol consumption
Participants were classified as non-drinkers, interim drinkers (<7 days/week), and daily drinkers, as previously described [18]. For the daily drinkers, we further collected information about the type of alcohol (hard liquor, wine, beer) as well as the amount of intake, which was calculated in grams per day by multiplying the average frequency (times per day) by the amount of each beverage and its corresponding pure ethanol content (5 g ethanol for every 100 g of beer, 12 g ethanol for every 100 g of wine, and 40 g for every 100 g of hard liquor) [12].
Assessment of sarcopenia
The status of sarcopenia was assessed every year. Muscle mass was measured by bioelectrical impedance analysis (BIA) (Inbody S10; Inbody Korea Ltd., Korea). The height-adjusted appendicular skeletal muscle mass index (ASMI) was calculated as ASM (the sum of skeletal muscle in the arms and legs) divided by height squared in meters (ASM/height [2]). Low muscle mass was defined as an ASMI <7.0 kg/m2 in men and <5.7 kg/m2 in women [8]. Muscle strength was represented by grip strength measured using a dynamometer (CAMRY EH101, China). Low muscle strength was defined as handgrip strength <28 kg in men and <18 kg in women [8]. Gait speed on a 6-m test <1 m/s was defined as declined physical performance [8].
Statistical analysis
Kolmogorov-Smirnov test was applied to test the normality of continuous variable. Non-normal data were represented as median and interquartile range (IQR), and compared by Mann-Whitney U test. Qualitative variables were represented as frequencies and compared by Pearson χ2 test. Logistic regression analyses were performed to identified the variables associated with the risk of sarcopenia. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. Dose-response analysis was performed by the method described by Greenland and Orsini [19, 20]. The ORs that were the most confounding factors were adjusted and their 95% CIs were used to estimate log ORs and their standard errors (SEs). Linear regression was used to explore the dose-response relationship of every 1g increase in daily alcohol intake with the aspects of sarcopenia. Restricted cubic splines (four knots at fixed percentiles of 5%, 35%, 65%, and 95% of the distribution) were applied to evaluate potential nonlinear dose-response relationship between alcohol consumption and sarcopenia. All statistical analyses were performed by using SPSS 28.0 (IBM SPSS, Inc., USA). All analyses were two-sided and P < 0.05 was considered as statistical significance.
Results
Baseline characteristics
Among 5244 enrolled subjects, there were 1,050 older adults drank alcohol (20.0%) with 35.9% in males and 4.4% in females. As shown in Table 1, the male drinkers had higher levels of aspartate transaminase (AST)/alanine transaminase (ALT) ratio and total bilirubin (TBIL), whereas the female drinkers had higher levels of hemoglobin (HGB) at baseline (P < 0.05). By contrast, there were no significant differences in other clinical parameters as well as the ASMI, grip strength, and gait speed between drinks and non-drinkers at baseline (P > 0.05).
TABLE 1
| Variables | Men | Women | ||||||
|---|---|---|---|---|---|---|---|---|
| Total (n = 2,603) | Non-drinker (n = 1,669) | Drinker (n = 934) | P | Total (n = 2,641) | Non-drinker (n = 2,525) | Drinker (n = 116) | P | |
| Age, y | 72 (69–76) | 73 (69–78) | 73 (68–77) | 0.861 | 73 (69–77) | 73 (69–77) | 72 (69–76) | 0.363 |
| BMI, kg/m2 | 23.5 ± 3.3 | 23.2 (20.9–25.6) | 23.3 (20.5–25.6) | 0.763 | 24.7 (22.4–27.2) | 24.7 (22.4–27.3) | 24.7 (22.5–26.7) | 0.637 |
| Waist-to-hip ratio | 0.90 (0.87–0.94) | 0.90 (0.86–0.94) | 0.90 (0.87–0.93) | 0.882 | 0.90 (0.86–0.94) | 0.90 (0.86–0.94) | 0.90 (0.87–0.93) | 0.630 |
| HGB, g/L | 137 (128–144) | 137 (126–142) | 138 (127–144) | 0.221 | 134 (127–139) | 133 (127–139) | 135 (129–142) | 0.013 |
| WBC, 109/L | 5.4 (4.6–6.4) | 5.4 (4.6–6.5) | 5.4 (4.6–6.4) | 0.486 | 5.3 (4.5–6.3) | 5.4 (4.5–6.3) | 5.2 (4.6–6.0) | 0.652 |
| PLT, 109/L | 139 (110–171) | 139 (109–170) | 140 (110–171) | 0.164 | 146 (116–181) | 146 (115–181) | 146 (116–182) | 0.560 |
| FBG, mmol/L | 5.4 (5.1–5.9) | 5.4 (5.1–5.9) | 5.4 (5.1–5.9) | 0.558 | 5.4 (5.1–6.0) | 5.4 (5.1–6.0) | 5.5 (5.2–5.9) | 0.762 |
| ALB, g/L | 37.2 (35.6–43.2) | 37.2 (35.3–43.9) | 37.3 (35.4–43.5) | 0.745 | 36.5 (34.2–41.2) | 36.2 (33.8–43.1) | 36.3 (33.4–42.8) | 0.152 |
| AST/ALT ratio | 1.48 (1.15–1.92) | 1.44 (1.13–1.86) | 1.57 (1.21–2.00) | <0.001 | 1.53 (1.20–1.96) | 1.52 (1.20–1.96) | 1.56 (1.25–1.96) | 0.479 |
| TBIL, μmol/L | 13.5 (10.6–17.7) | 13.2 (10.4–17.2) | 14.5 (11.0–19.0) | <0.001 | 11.8 (9.3–15.1) | 11.8 (9.3–15.1) | 11.8 (9.2–15.0) | 0.780 |
| Cr, μmol/L | 64.1 (53.1–75.1) | 64.2 (53.5–76.2) | 64.1 (52.3–75.6) | 0.110 | 62.5 (53.9–71.6) | 62.6 (54.0–71.8) | 62.1 (53.8–70.7) | 0.413 |
| BUN, mmol/L | 5.3 (4.4–6.6) | 5.3 (4.5–6.6) | 5.4 (4.4–6.7) | 0.683 | 5.3 (4.4–6.4) | 5.3 (4.4–6.4) | 5.5 (4.5–6.5) | 0.184 |
| TC, mmol/L | 4.6 (4.1–5.2) | 4.6 (4.1–5.1) | 4.6 (4.2–5.0) | 0.416 | 4.5 (4.1–5.1) | 4.5 (4.0–5.1) | 5.6 (4.1–5.3) | 0.109 |
| TG, mmol/L | 1.1 (0.9–1.6) | 1.1 (0.9–1.5) | 1.1 (0.8–1.6) | 0.775 | 1.2 (1.1–1.4) | 1.2 (1.1–1.5) | 1.2 (1.0–1.4) | 0.813 |
| LDL-C, mmol/L | 1.93 (1.55–2.32) | 1.93 (1.59–2.33) | 1.92 (1.58–2.32) | 0.245 | 2.18 (1.78–2.58) | 2.17 (1.78–2.58) | 2.25 (1.82–2.63) | 0.304 |
| ASMI, kg/m2 | 7.7 (7.1–8.8) | 7.6 (7.1–8.9) | 7.7 (7.2–8.9) | 0.823 | 6.1 (5.6–6.7) | 6.2 (5.6–6.7) | 6.1 (5.7–6.6) | 0.954 |
| Grip strength, kg | 34.2 (32.3–38.3) | 33.6 (31.8–39.8) | 34.1 (32.5–40.0) | 0.572 | 20.9 (18.0–23.8) | 20.8 (18.0–23.8) | 21.4 (19.2–23.5) | 0.181 |
| Gait speed, m/s | 1.36 (1.06–1.56) | 1.35 (1.04–1.65) | 1.38 (1.03–1.77) | 0.221 | 1.19 (1.09–1.29) | 1.19 (1.09–1.30) | 1.18 (1.04–1.30) | 0.131 |
Characteristics according to alcohol consumption at baseline.
BMI, body mass index; ALB, albumin; ASMI, appendicular muscle mass index; HGB, hemoglobin; WBC, white blood cell; PLT, platelet; FBG, fasting blood glucose; AST, aspartate transaminase; ALT alanine transaminase; TBIL, total bilirubin; Cr, creatinine; BUN, blood urea nitrogen; TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol.
Association of alcohol consumption with sarcopenia
After a follow-up of 4 years, there were 691 aged males had sarcopenia (26.5%), with 184 patients in the drinking group and 507 patients in the non-drinking group. In addition, there were 527 aged females had sarcopenia (20.0%), with 11 patients in the drinking group and 516 patients in the non-drinking group. Interestingly, drinkers had a lower incidence of sarcopenia than those non-drinkers both in males (19.7% vs. 30.4%, P < 0.001) and females (9.5% vs. 20.4%, P < 0.001). Moreover, male drinkers also had higher levels of muscle mass [median (IQR): 7.3 (6.7–7.9) kg/m2 vs. 7.1 (6.5–7.7) kg/m2, P < 0.001], grip strength [median (IQR): 31.1 (26.5–35.0) kg vs. 29.6 (24.8–38.8) kg, P < 0.001], and gait speed [median (IQR): 1.08 (0.98–1.17) m/s vs. 1.05 (0.94–1.15) m/s, P < 0.001] than those non-drinkers (Figures 1A–C). By contrast, female drinkers had higher gait speed [median (IQR): 1.02 (0.94–1.11) m/s vs. 0.99 (0.89–1.09) m/s, P = 0.031], with no significant difference of muscle mass [median (IQR): 6.0 (5.2–6.4) kg/m2 vs. 6.1 (5.2–6.7) kg/m2, P = 0.827] and grip strength [median (IQR): 19.1 (18.3–21.0) kg vs. 18.6 (17.8–22.3) kg, P = 0.103] when compared to those female non-drinkers (Figures 1D–F).
FIGURE 1
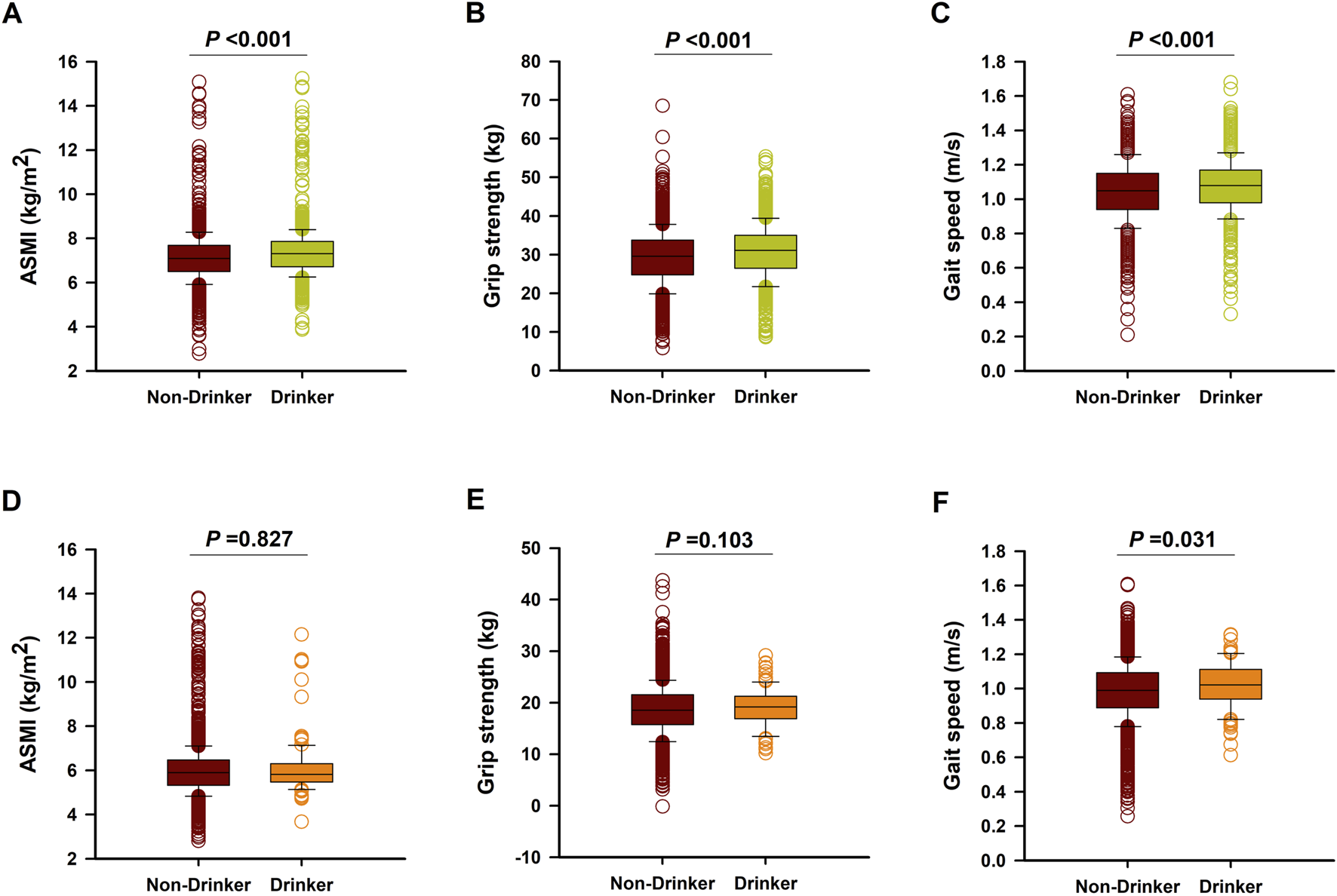
The levels of ASMI, grip strength, and gait speed in male (A–C) and Female (D–F) non-drinkers and drinkers after 4-years follow-up.
Univariate logistic analysis indicated that the factors correlated with the risk of sarcopenia in males included age, low levels of body mass index (BMI), HGB, white blood counts (WBC), fasting blood glucose (FBG), TBIL, triglyceride (TG), and low-density lipoprotein cholesterol (LDL-C), but higher AST/ALT ratio and high-density lipoprotein cholesterol (HDL-C) (Table 2). Importantly, compared with non-drinkers, older men with interim drinking (RR = 0.56; 95%CI = 0.38–0.81; P = 0.003) and daily drinking (RR = 0.56; 95%CI = 0.46–0.69; P < 0.001) were less likely to have sarcopenia. For aged females, univariate logistic analysis indicated that the risks factors of sarcopenia included age, low levels of BMI, HGB, FBG, and TG, but higher AST/ALT ratio and HDL-C. Compared with non-drinkers, older women with daily drinking (RR = 0.35; 95%CI = 0.14–0.88; P = 0.024) were less likely to suffer from sarcopenia (Table 2). However, these protective effects of drinking on the incidence of sarcopenia were lost after adjustment for potential confounding factors (Table 3). Subgroup analysis suggested that in the older adults with age <85 years old, both interim drinking (RR = 0.60; 95%CI = 0.39–0.93; P = 0.021 for males; RR = 0.36; 95%CI = 0.13–0.90; P = 0.035 for females) and daily drinking (RR = 0.78; 95%CI = 0.61–0.99; P = 0.045 for males; RR = 0.34; 95%CI = 0.12–0.96; P = 0.041 for females) were correlated with decreased risk of sarcopenia even after adjustment for confounding factors. Moreover, daily alcohol consumption (RR = 0.75; 95%CI = 0.58–0.95; P = 0.019) was also related to decreased risk of sarcopenia in aged males who were not underweight (BMI ≥18.5 kg/m2) (Table 3).
TABLE 2
| Variables | Men | Women | ||||
|---|---|---|---|---|---|---|
| β | OR (95%CI) | P | β | OR (95%CI) | P | |
| Age | 0.141 | 1.15 (1.13–1.17) | <0.001 | 0.143 | 1.15 (1.13–1.18) | <0.001 |
| BMI | −0.264 | 0.77 (0.74–0.79) | <0.001 | −0.296 | 0.74 (0.72–0.77) | <0.001 |
| HGB | −0.035 | 0.97 (0.96–0.97) | <0.001 | −0.028 | 0.97 (0.96–0.98) | <0.001 |
| WBC | −0.090 | 0.91 (0.86–0.97) | 0.003 | −0.037 | 0.96 (0.90–1.03) | 0.268 |
| PLT | −0.001 | 1.00 (1.00–1.00) | 0.302 | −0.001 | 1.00 (1.00–1.00) | 0.135 |
| FBG | −0.098 | 0.91 (0.84–0.98) | 0.013 | −0.077 | 0.93 (0.86–0.99) | 0.041 |
| ALB | 0.003 | 0.98 (0.97–1.03) | 0.546 | 0.008 | 1.02 (0.98–1.05) | 0.411 |
| AST/ALT ratio | 0.438 | 1.55 (1.38–1.74) | <0.001 | 0.645 | 1.91 (1.67–2.17) | <0.001 |
| TBIL | −0.019 | 0.98 (0.97–0.99) | 0.008 | −0.009 | 0.99 (0.97–1.01) | 0.340 |
| Cr | 0.001 | 1.00 (1.00–1.00) | 0.617 | 0.003 | 1.00 (1.00–1.00) | 0.261 |
| BUN | 0.034 | 1.03 (0.98–1.08) | 0.143 | 0.063 | 1.07 (1.01–1.13) | 0.029 |
| TC | −0.058 | 0.94 (0.87–1.03) | 0.170 | 0.011 | 1.01 (0.93–1.10) | 0.790 |
| TG | −0.339 | 0.71 (0.62–0.82) | <0.001 | −0.241 | 0.79 (0.70–0.88) | <0.001 |
| LDL-C | −0.159 | 0.85 (0.74–0.99) | 0.036 | −0.095 | 0.91 (0.78–1.06) | 0.220 |
| HDL-C | 0.575 | 1.78 (1.42–2.23) | <0.001 | 0.868 | 2.38 (1.82–3.12) | <0.001 |
| Alcohol consumption | ||||||
| Non-drinker (64.12%) | 1 (reference) | 1 (reference) | ||||
| Interim drinker (7.07%) | −0.584 | 0.56 (0.38–0.81) | 0.003 | −0.741 | 0.48 (0.20–1.12) | 0.089 |
| Daily drinker (28.81%) | −0.574 | 0.56 (0.46–0.69) | <0.001 | −1.057 | 0.35 (0.14–0.88) | 0.024 |
Univariate logistic analysis for risk factors of sarcopenia in older adults.
OR, odds ratio; CI, confidence interval; BMI, body mass index; HGB, hemoglobin; WBC, white blood cell; PLT, platelet; FBG, fasting blood glucose; ALB, albumin; AST, aspartate transaminase; ALT alanine transaminase; TBIL, total bilirubin; Cr, creatinine; BUN, blood urea nitrogen; TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol.
TABLE 3
| Variables | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| β | OR (95%CI) | p | β | OR (95%CI) | p | |
| Men | ||||||
| Overall analyses | ||||||
| Non-drinker (64.12%) | 1 (reference) | 1 (reference) | ||||
| Interim drinker (7.07%) | −0.283 | 0.75 (0.50–1.15) | 0.187 | −0.249 | 0.78 (0.51–1.19) | 0.249 |
| Daily drinker (28.81%) | −0.225 | 0.80 (0.63–1.01) | 0.058 | −0.150 | 0.86 (0.67–1.10) | 0.236 |
| Stratification analyses | ||||||
| Age | ||||||
| <85 years (n = 2,489) | ||||||
| Non-drinker (63.08%) | 1 (reference) | 1 (reference) | ||||
| Interim drinker (7.15%) | −0.562 | 0.57 (0.38–0.87) | 0.009 | −0.504 | 0.60 (0.39–0.93) | 0.021 |
| Daily drinker (29.77%) | −0.383 | 0.68 (0.55–0.85) | 0.001 | −0.247 | 0.78 (0.61–0.99) | 0.045 |
| ≥85 years (n = 114) | ||||||
| Non-drinker (86.84%) | 1 (reference) | 1 (reference) | ||||
| Interim drinker (5.26%) | 1.154 | 3.17 (0.30–33.57) | 0.338 | 1.147 | 3.15 (0.18–54.19) | 0.430 |
| Daily drinker (7.89%) | −0.721 | 0.49 (0.11–2.20) | 0.349 | −1.109 | 0.33 (0.05–2.26) | 0.259 |
| BMI | ||||||
| <18.5 kg/m2 (n = 164) | ||||||
| Non-drinker (81.71%) | 1 (reference) | 1 (reference) | ||||
| Interim drinker (4.27%) | −1.029 | 0.36 (0.07–1.79) | 0.211 | −1.240 | 0.29 (0.05–1.72) | 0.172 |
| Daily drinker (14.02%) | −0.553 | 0.58 (0.23–1.46) | 0.245 | −0.264 | 0.77 (0.24–2.44) | 0.654 |
| ≥18.5 kg/m2 (n = 2,439) | ||||||
| Non-drinker (62.94%) | 1 (reference) | 1 (reference) | ||||
| Interim drinker (7.26%) | −0.221 | 0.80 (0.53–1.21) | 0.296 | −0.217 | 0.81 (0.53–1.23) | 0.314 |
| Daily drinker (29.81%) | −0.240 | 0.79 (0.63–0.99) | 0.040 | −0.294 | 0.75 (0.58–0.95) | 0.019 |
| Women | ||||||
| Overall analyses | ||||||
| Non-drinker (95.61%) | 1 (reference) | 1 (reference) | ||||
| Interim drinker (2.08%) | −0.935 | 0.39 (0.16–1.01) | 0.057 | −0.937 | 0.39 (0.15–1.01) | 0.059 |
| Daily drinker (2.31%) | −0.951 | 0.39 (0.14–1.04) | 0.061 | −0.886 | 0.41 (0.15–1.11) | 0.080 |
| Stratification analyses | ||||||
| Age | ||||||
| <85 years (n = 2,511) | ||||||
| Non-drinker (95.58%) | 1 (reference) | 1 (reference) | ||||
| Interim drinker (2.07%) | −1.052 | 0.35 (0.12–0.99) | 0.048 | −1.022 | 0.36 (0.13–0.90) | 0.035 |
| Daily drinker (2.35%) | −1.135 | 0.32 (0.11–0.91) | 0.033 | −1.089 | 0.34 (0.12–0.96) | 0.041 |
| ≥85 years (n = 130) | ||||||
| Non-drinker (96.15%) | 1 (reference) | 1 (reference) | ||||
| Interim drinker (2.31%) | 0.385 | 1.47 (0.13–16.93) | 0.758 | 0.248 | 1.28 (0.09–18.10) | 0.854 |
| Daily drinker (1.54%) | −0.616 | 0.54 (0.03–9.00) | 0.668 | −0.443 | 0.64 (0.03–14.11) | 0.779 |
| BMI | ||||||
| <18.5 kg/m2 (n = 111) | ||||||
| Never drinker (99.10%) | - | - | - | - | - | - |
| Interim drinker (0.90%) | - | - | - | - | - | - |
| Daily drinker (0.00%) | - | - | - | - | - | - |
| ≥18.5 kg/m2 (2,530) | ||||||
| Never drinker (95.45%) | 1 (reference) | 1 (reference) | ||||
| Interim drinker (2.13%) | −0.701 | 0.50 (0.21–1.20) | 0.120 | −0.816 | 0.44 (0.18–1.09) | 0.075 |
| Daily drinker (2.41%) | −0.841 | 0.43 (0.17–1.12) | 0.085 | −0.855 | 0.43 (0.16–1.11) | 0.080 |
Multivariate logistic regression analyses for the association of alcohol consumption with sarcopenia.
OR, odds ratio; CI, confidence interval; BMI, body mass index.
Model 1: Adjusted for age (for overall analyses and BMI stratification analyses) or BMI (for overall analyses and age stratification analyses).
Model 2: Adjusted for age (for overall analyses and BMI stratification analyses) or BMI (for overall analyses and age stratification analyses), HGB, WBC, PLT, FBG, AST/ALT ratio, TBIL, Cr, BUN, TC, TG, LDL-C, and HDL-C.
Dose-response relationship between daily alcohol consumption and sarcopenia
We further analyzed the dose-response relationship between daily alcohol consumption and sarcopenia. Considering the small number of daily drinkers (n = 61) in the females, we only performed dose-response analysis in the male daily drinkers (n = 750). As shown in Figure 2, restricted cubic splines model indicated no nonlinear dose-response relationship between daily alcohol intake and risk of sarcopenia (χ2 = 1.76, P = 0.414), low muscle mass (χ2 = 3.68, P = 0.159), low grip strength (χ2 = 2.38, P = 0.304), and low gait speed (χ2 = 0.55, P = 0.760). Unfortunately, we did not find linear dose-response relationship between daily alcohol intake and the risk of sarcopenia (P = 0.423), as well as low muscle mass (P = 0.297), low grip strength (P = 0.460), and low gait speed (P = 0.156).
FIGURE 2
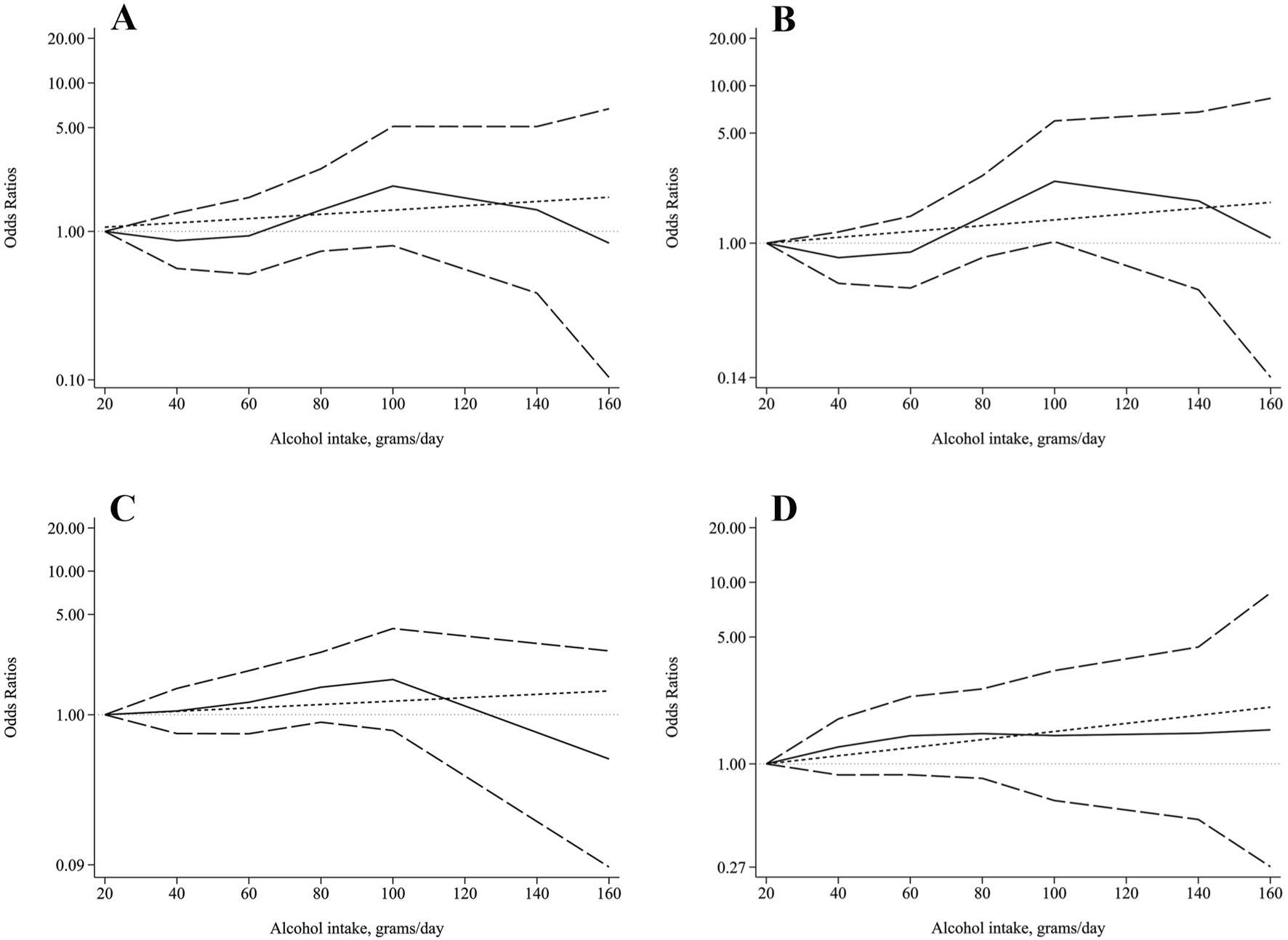
Dose-response relationship between alcohol intake and (A) sarcopenia, (B) low AMSI, (C) low grip strength, and (D) low gait speed. The solid line and the long dash line represent the ORs and its 95%CI. Short dash line represents the linear relationship. Linear and spline models were adjusted for age, BMI, HGB, WBC, PLT, FBG, AST/ALT ratio, TBIL, Cr, BUN, TC, TG, LDL-C, and HDL-C.
Discussion
Along with the population aging, sarcopenia has been getting more and more attention worldwide. Although various types of lifestyles have been linked to the etiology of sarcopenia [21]; however, the exact correlation between alcohol beverage consumption and sarcopenia remains controversial. Interestingly, we here showed that alcohol drinking might have a potential beneficial effect on the risk of sarcopenia in a community-dwelling population in Yangzhou city of older adults, especially in those younger than 85 years old. Further studies will be needed to identified the appropriate amount of alcohol consumption for the prevention of sarcopenia.
Alcohol consumption is growing globally and accounts for about 5.1% of the global burden of human diseases, especially cardiovascular disease and cancer [22–24]. Animal studies have demonstrated that alcohol can inhibit the synthesis of protein predominately in type II muscle fibers which in turn causes muscle atrophy [25–28]. Moreover, ethanol exposure also induces autophagy flux in C2C12 myotubes, which contributes to the pathogenesis of sarcopenia [26]. However, previous retrospective studies showed controversial results on the relationship between alcohol consumption and sarcopenia. Some studies found detrimental effects of alcohol on the risk of sarcopenia [10–12, 29, 30], whereas others reported negative results between two of them [31–34]. Although the different types of alcoholic beverages, the amount of consumption, as well as the different drinking patterns may affect the relationship between drinking and human diseases [35], the observed protective effects of alcohol intake on the risk of sarcopenia in our present study should be interpreted with caution. According to the dietary guidelines for Chinese residents, the recommended amount of alcohol intake should less than 25g/day [36]. Particularly, daily drinking was associated with lower risk of sarcopenia only in older adults younger than 85 years. This age difference in the relationship between alcohol consumption and sarcopenia may be attribute to the decreased alcohol intake along with aging [37]. Moreover, drinking pattern, such as whether the alcohol is taken at mealtimes [38], may also influence the effect of alcohol consumption on sarcopenia. Interestingly, a previous study showed that drinking with meals might be beneficial for decreasing the all-cause mortality [39]. However, we did not find a significant dose-response relationship between alcohol intake and sarcopenia. Considering that the individual dose was obtained by self-report, the limitation in measurement precision could obscure such a relationship. Therefore, further studies focused on different drinking pattern will help us to understand the potential health effects of alcohol on sarcopenia.
There are some potential explanations that may account for the protection effects against sarcopenia by alcohol consumption. Since malnutrition, especially with low BMI (<18.5 kg/cm2), has been demonstrated as one of the most important risk factors for sarcopenia [7], the leading possibility might be related to the weight gain in alcohol consumers, especially in those daily drinkers [40]. However, considering that weight gain induced by alcohol consumption primarily due to an increase in fat mass, which often contributes to obesity, further studies are needed to confirm the association of drinking with the detailed amount of body composition. Another potential reason may be related the relatively positive life attitude of people who drink moderate amounts of alcohol [41]. In the present study, older adults with interim drinking also had a lower risk of sarcopenia. These people may have more social intercourse and exercise, both of which are important preventive measures for the management of sarcopenia [7]. Moreover, another study reported that alcohol consumption could also increase the level of HDL-C [42], which is inversely correlated with the risk of sarcopenia [43]. Sphingolipids level is also decreased in drinkers when compared to those non-drinkers [44]. As an important bioactive sphingolipid, previous studies have shown that ceramide can directly activate atypical PKC isoform protein kinase Cζ to induce endoplasmic reticulum stress and mitochondrial dysfunction [45], which play crucial roles in the pathogenesis of sarcopenia [46]. Therefore, moderate alcohol may play a protective role in skeletal muscle by depleting sphingolipids, especially ceramide. Nevertheless, further in‐depth experiments are needed to investigate the explicit mechanism governing the effects of different types and amount of alcohol on the development of sarcopenia.
Several limitations should still be considered in the present study. Firstly, the information of alcohol consumption was self-reported, which may cause social desirability bias and recall error. Future prospective studies with an intervention of different amount of alcohol will help to determine the dose-dependent effects of alcohol consumption on the prevention of sarcopenia. Secondly, almost all the older adults in this study consumed liquor. The effects of different types of alcohol beverages such as wine, beer, sake, yellow rice, etc. on the risk of sarcopenia will need to be assessed. Thirdly, we cannot rule out the possibility of other potential confounding dietary factors, such as the nutritional status, physical activity, and socio-economic conditions as well as the consumption of tea, coffee, fruits, vitamins or other antioxidant substances.
Conclusion
In summary, our findings suggest that alcohol intake may not be a risk factor for the development of sarcopenia in this Yangzhou cohort. Instead, appropriate amount of alcohol consumption might be related to decreased risk of sarcopenia in older adults younger than 85 years. However, heavy drinking has significant social burden in addition to human health and therefore is not recommended. Advices regarding the health effects of alcohol drinking on sarcopenia need to be further individualized according to the specific medical status.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Ethics Committee of Sir Run Run Hospital, Nanjing Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
WG, Z-KS, and X-LY contributed to the conception and design of the study. E-HM, Y-LB, Q-LL, and J-SX contributed to data acquisition. Y-LB, E-HM, and Z-KS analyzed the data. E-HM and Y-LB drafted the manuscript. WG, Q-LL, and X-LY revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by grants from the National Key Research and Development Plan of China (No. 2020YFC2008505 to XL), the National Natural Science Foundation of China (No. 81970217 to WG), and the Jiangsu Commission of Health (No. LKZ2023005 to WG, No. LKM2023004 to Z-KS), the project of Zhongda Hospital Affiliated to Southeast University for cultivating academic talent (No. CZXM-GSPRC22 to WG), the Zhongda Hospital Affiliated to Southeast University, Jiangsu Province High-Level Hospital Pairing Assistance Construction Funds (No. ZDLYG10 to WG), the Zhongda Hospital Affiliated to Southeast University, Jiangsu Province High-Level Hospital Construction Funds (No. GSP-LCYJFH17 to WG), China Heart House-Chinese Cardiovascular Association HX fund (No. 2022-CCA-HX-082 to WG), Bethune Charitable Foundation (No. 2024020185BA to WG).
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
References
1.
Cruz-Jentoft AJ Bahat G Bauer J Boirie Y Bruyere O Cederholm T et al Sarcopenia: revised European consensus on definition and diagnosis. Age and Ageing (2019) 48:16–31. 10.1093/ageing/afy169
2.
Shafiee G Keshtkar A Soltani A Ahadi Z Larijani B Heshmat R . Prevalence of sarcopenia in the world: a systematic review and meta- analysis of general population studies. J Diabetes Metab Disord (2017) 16:21. 10.1186/s40200-017-0302-x
3.
Du Y Wang X Xie H Zheng S Wu X Zhu X et al Sex differences in the prevalence and adverse outcomes of sarcopenia and sarcopenic obesity in community dwelling elderly in East China using the AWGS criteria. BMC Endocr Disord (2019) 19:109. 10.1186/s12902-019-0432-x
4.
Meng P Hu YX Fan L Zhang Y Zhang MX Sun J et al Sarcopenia and sarcopenic obesity among men aged 80 years and older in Beijing: prevalence and its association with functional performance. Geriatr and Gerontol Int (2014) 14(Suppl. 1):29–35. 10.1111/ggi.12211
5.
Yang Q Vijayakumar A Kahn BB . Metabolites as regulators of insulin sensitivity and metabolism. Nat Rev Mol Cel Biol (2018) 19:654–72. 10.1038/s41580-018-0044-8
6.
Zeng Y Hu X Xie L Han Z Zuo Y Yang M . The prevalence of sarcopenia in Chinese elderly nursing home residents: a comparison of 4 diagnostic criteria. J Am Med Directors Assoc (2018) 19:690–5. 10.1016/j.jamda.2018.04.015
7.
Cruz-Jentoft AJ Sayer AA . Sarcopenia. The Lancet (2019) 393:2636–46. 10.1016/s0140-6736(19)31138-9
8.
Chen LK Woo J Assantachai P Auyeung TW Chou MY Iijima K et al Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Directors Assoc (2020) 21:300–7.e2. 10.1016/j.jamda.2019.12.012
9.
Yaru L Jing W Liyun Z Zhihong W Dongmei Y Yuna H et al The drinking status and associated factors in adults in China Chinese Journal of Epidemiology (2018);39:898–903.
10.
Gabat JAL Faltado AL Jr Sedurante MB Tee ML . Association of obesity and sarcopenia among adult Filipinos. Osteoporos sarcopenia (2018) 4:109–13. 10.1016/j.afos.2018.08.001
11.
Pang BWJ Wee SL Lau LK Jabbar KA Seah WT Ng DHM et al Prevalence and associated factors of sarcopenia in Singaporean adults-the yishun study. J Am Med Directors Assoc (2021) 22:885.e1–885.e10. 10.1016/j.jamda.2020.05.029
12.
Zhai J Ma B Qin J Lyu Q Khatun P Liang R et al Alcohol consumption patterns and the risk of sarcopenia: a population-based cross-sectional study among Chinese women and men from Henan province. BMC public health (2022) 22:1894. 10.1186/s12889-022-14275-6
13.
Hai S Wang H Cao L Liu P Zhou J Yang Y et al Association between sarcopenia with lifestyle and family function among community-dwelling Chinese aged 60 years and older. BMC Geriatr (2017) 17:187. 10.1186/s12877-017-0587-0
14.
Steffl M Bohannon RW Petr M Kohlikova E Holmerova I . Alcohol consumption as a risk factor for sarcopenia - a meta-analysis. BMC Geriatr (2016) 16:99. 10.1186/s12877-016-0270-x
15.
Bu YL Wang C Zhao C Lu X Gao W . The association of alcohol consumption with the risk of sarcopenia: a dose-response meta-analysis. The Am J Drug Alcohol Abuse (2024) 50:305–20. 10.1080/00952990.2023.2300049
16.
Hong SH Bae YJ . Association between alcohol consumption and the risk of sarcopenia: a systematic review and meta-analysis. Nutrients (2022) 14:3266. 10.3390/nu14163266
17.
Anonymous. Code of ethics on human experimentation adapted from the Helsinki declaration of the world medical association. The Am J orthopsychiatry (1968) 38:589–90. 10.1111/j.1939-0025.1968.tb02426.x
18.
Lau EM Lynn HS Woo JW Kwok TC Melton LJ 3rd . Prevalence of and risk factors for sarcopenia in elderly Chinese men and women. The Journals Gerontol Ser A: Biol Sci Med Sci (2005) 60:213–6. 10.1093/gerona/60.2.213
19.
Greenland S Longnecker MP . Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol (1992) 135:1301–9. 10.1093/oxfordjournals.aje.a116237
20.
Orsini N Bellocco R Greenland S . Generalized least squares for trend estimation of summarized dose-response data. The Stata J Promoting Commun Stat Stata (2006) 6:40–57. 10.1177/1536867x0600600103
21.
Gao Q Hu K Yan C Zhao B Mei F Chen F et al Associated factors of sarcopenia in community-dwelling older adults: a systematic review and meta-analysis. Nutrients (2021) 13:4291. 10.3390/nu13124291
22.
Aberg F Byrne CD Pirola CJ Mannisto V Sookoian S . Alcohol consumption and metabolic syndrome: clinical and epidemiological impact on liver disease. J Hepatol (2023) 78:191–206. 10.1016/j.jhep.2022.08.030
23.
Monteiro MG . Alcohol consumption: overview of international trends. Reference Module Biomed Sci (2019). 10.1016/B978-0-12-801238-3.03186-X
24.
Organization W . Global status report on noncommunicable diseases 2014 (2014);
25.
Steiner JL Lang CH . Alcohol impairs skeletal muscle protein synthesis and mTOR signaling in a time-dependent manner following electrically stimulated muscle contraction. J Appl Physiol (2014) 117:1170–9. 10.1152/japplphysiol.00180.2014
26.
Thapaliya S Runkana A McMullen MR Nagy LE McDonald C Prasad SVN et al Alcohol-induced autophagy contributes to loss in skeletal muscle mass. Autophagy (2014) 10:677–90. 10.4161/auto.27918
27.
Lang CH Frost RA Svanberg E Vary TC . IGF-I/IGFBP-3 ameliorates alterations in protein synthesis, eIF4E availability, and myostatin in alcohol-fed rats. Am J Physiol Endocrinol Metab (2004) 286:E916–26. 10.1152/ajpendo.00554.2003
28.
Lang CH Pruznak AM Nystrom GJ Vary TC . Alcohol-induced decrease in muscle protein synthesis associated with increased binding of mTOR and raptor: comparable effects in young and mature rats. Nutr and Metab (2009) 6:4. 10.1186/1743-7075-6-4
29.
Prokopidis K Witard OC . Understanding the role of smoking and chronic excess alcohol consumption on reduced caloric intake and the development of sarcopenia. Nutr Res Rev (2022) 35:197–206. 10.1017/s0954422421000135
30.
Fanelli Kuczmarski M Mason MA Beydoun MA Allegro D Zonderman AB Evans MK . Dietary patterns and sarcopenia in an urban African American and White population in the United States. J Nutr Gerontol Geriatr (2013) 32:291–316. 10.1080/21551197.2013.840255
31.
Ko YC Chie WC Wu TY Ho CY Yu WR . A cross-sectional study about the relationship between physical activity and sarcopenia in Taiwanese older adults. Scientific Rep (2021) 11:11488. 10.1038/s41598-021-90869-1
32.
Daskalopoulou C Wu YT Pan W Gine Vazquez I Prince M Prina M et al Factors related with sarcopenia and sarcopenic obesity among low- and middle-income settings: the 10/66 DRG study. Scientific Rep (2020) 10:20453. 10.1038/s41598-020-76575-4
33.
Castillo EM Goodman-Gruen D Kritz-Silverstein D Morton DJ Wingard DL Barrett-Connor E . Sarcopenia in elderly men and women: the Rancho Bernardo study. Am J Prev Med (2003) 25:226–31. 10.1016/s0749-3797(03)00197-1
34.
Akune T Muraki S Oka H Tanaka S Kawaguchi H Nakamura K et al Exercise habits during middle age are associated with lower prevalence of sarcopenia: the ROAD study. Osteoporos Int (2014) 25:1081–8. 10.1007/s00198-013-2550-z
35.
Hendriks HFJ . Alcohol and human health: what is the evidence?Annu Rev Food Sci Technol (2020) 11:1–21. 10.1146/annurev-food-032519-051827
36.
Wang SS Lay S Yu HN Shen SR . Dietary guidelines for Chinese residents (2016): comments and comparisons. J Zhejiang Univ Sci B (2016) 17:649–56. 10.1631/jzus.b1600341
37.
Ferreira MP Weems MK . Alcohol consumption by aging adults in the United States: health benefits and detriments. J Am Diet Assoc (2008) 108:1668–76. 10.1016/j.jada.2008.07.011
38.
Valencia-Martin JL Galan I Rodriguez-Artalejo F . The association between alcohol consumption patterns and adherence to food consumption guidelines. Alcohol Clin Exp Res (2011) 35:2075–81. 10.1111/j.1530-0277.2011.01559.x
39.
Trevisan M Schisterman E Mennotti A Farchi G Conti S . Drinking pattern and mortality:. Ann Epidemiol (2001) 11:312–9. 10.1016/s1047-2797(00)00183-6
40.
Traversy G Chaput JP . Alcohol consumption and obesity: an update. Curr Obes Rep (2015) 4:122–30. 10.1007/s13679-014-0129-4
41.
French MT Norton EC Fang H Maclean JC . Alcohol consumption and body weight. Health Econ (2010) 19:814–32. 10.1002/hec.1521
42.
Gaziano JM Buring JE Breslow JL Goldhaber SZ Rosner B VanDenburgh M et al Moderate alcohol intake, increased levels of high-density lipoprotein and its subfractions, and decreased risk of myocardial infarction. New Engl J Med (1993) 329:1829–34. 10.1056/nejm199312163292501
43.
Damayanthi H Moy FM Abdullah KL Dharmaratne SD . Health related quality of life and its associated factors among community-dwelling older people in Sri Lanka: a cross-sectional study. Arch Gerontol Geriatr (2018) 76:215–20. 10.1016/j.archger.2018.03.009
44.
Zhao X Zhou R Li H Fan Y Sun Y Hu X et al The effects of moderate alcohol consumption on circulating metabolites and gut microbiota in patients with coronary artery disease. Front Cardiovasc Med (2021) 8:767692. 10.3389/fcvm.2021.767692
45.
Chavez JA Summers SA . A ceramide-centric view of insulin resistance. Cell Metab (2012) 15:585–94. 10.1016/j.cmet.2012.04.002
46.
Hoogkamer W . Perception of gait asymmetry during split-belt walking. Exerc Sport Sci Rev (2017) 45:34–40. 10.1249/jes.0000000000000094
Summary
Keywords
alcohol, sarcopenia, older adults, community-dwelling, odds ratio
Citation
Mao E-H, Bu Y-L, Liu Q-L, Xu J-S, Lu X, Yang X-L, Gao W and Shen Z-K (2025) Alcohol consumption may not be a risk factor for sarcopenia in the older adults. Exp. Biol. Med. 250:10520. doi: 10.3389/ebm.2025.10520
Received
07 February 2025
Accepted
20 May 2025
Published
29 May 2025
Volume
250 - 2025
Updates
Copyright
© 2025 Mao, Bu, Liu, Xu, Lu, Yang, Gao and Shen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xi-Lan Yang, xilanyang@njmu.edu.cn; Wei Gao, drweig1984@outlook.com; Zheng-Kai Shen, shenzhengkai@jscdc.cn
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.