Abstract
The relationship between the systemic immune-inflammation index (SII) and the risk of developing cardiovascular disease (CVD) over the next 10 years in the United States is largely unknown. The aim of this study is to assess the association between SII and 10-year CVD risk. This population-based cross-sectional study included 9901 participants aged between 30 and 74 from the National Health and Nutrition Examination Survey (NHANES) 1999–2018. The 10-year CVD risk was calculated using the Framingham cardiovascular risk score (FRS). The Pearson test, generalized linear model (GLM) and restricted cubic splines (RCS) were used to analyze the associations between SII and the FRS. Based on the total population, the Pearson test and GLM revealed that there were positive relationships between Ln-transformed SII (Ln (SII)) and the FRS. After adjusting for confounding factors, the odds ratio (OR) for the FRS was 1.52 (95% confidence interval [CI]: 1.12–2.06) per unit increment in Ln (SII) (P = 0.009). Compared to the lowest quartile (Q1) of Ln (SII), the OR for the FRS in the highest quartile (Q4) was 1.89 (95% CI: 1.20–2.98; P = 0.007). RCS revealed that there was a linear association between Ln (SII) and the FRS (P for non-linearity = 0.972). As Ln (SII) increased, the value of FRS rose gradually (P for overall trend <0.001). However, the relationship between Ln (SII) and FRS showed ethnic heterogeneity. In conclusion, SII exhibits significant associations with 10-year CVD risk as assessed by the FRS. However, this association varies across ethnic groups, necessitating cautious application and further validation.
Impact statement
This study investigated the relationship between systemic immune-inflammation index (SII) and cardiovascular disease (CVD) risk. The 10-year CVD risk was calculated by Framingham cardiovascular risk scores (FRS). We found that there was a positive significant association between SII and 10-year CVD risk. Therefore, SII is expected to become an effective metric for identifying the 10-year CVD risk of human, providing well preventive strategies and improving risk stratification.
Introduction
Cardiovascular disease (CVD) is one of the most significant public health problems threatening human life. In recent years, it has remained the leading cause of death and disability worldwide in addition to being the leading cause of disease burden in the United States [1]. A report from the American Heart Association suggested that the prevalence of CVD is 48.6% in adults aged ≥20 years. Moreover, in 2020 the number of cardiovascular deaths worldwide was 19.05 million, which amounted to an increase of nearly one-fifth since 2010 [2]. Dyslipidemia, hypercoagulability, insulin resistance, hypertension, and inflammatory responses are the risk factors for the pathogenesis of CVD [3]. Based on several sex-specific multivariable risk factors, the Framingham Heart Study developed the first CVD risk equations, which were used to quantify risk and guide preventive care [4]. The Framingham Risk Score (FRS) is a widely used predictive tool that can be applied to relatively healthy individuals to estimate their probability of having a fatal or non-fatal cardiovascular event over the next decade [5, 6], and it has been used in several studies [6–8]. In addition, in recent years, inflammatory cytokines have shown promise as diagnostic tools for coronary heart disease, heart failure (HF), and other CVDs [9, 10].
With the in-depth study of chronic systemic inflammation, it has been found that inflammatory responses are connected to many different diseases [11–14]. Several immune cell types and inflammatory mediators have been implicated in the progression and pathogenesis of CVD [15, 16]. Mounting evidence suggests that chronic inflammation substantially contributes to cardiovascular risk. The Systemic Immune-Inflammation Index (SII), which is calculated using platelet, neutrophil, and lymphocyte counts, is a comprehensive biomarker that reflects both local immune responses and systemic inflammation [17]. Mechanistically, SII captures key inflammatory processes across atherogenesis. In the early phase, hemodynamic stress and lipid abnormalities appear to trigger inflammatory activation in endothelial cells, facilitating monocyte recruitment through adhesion molecules [18]. In the advanced phase, macrophage-derived inflammatory mediators promote extracellular matrix degradation via matrix metalloproteinases, increasing plaque vulnerability [19, 20]. Although SII has been established as an independent predictor of cancer, CVD and all-cause mortality [21–26], its association with the FRS — a key CVD risk assessment tool — remains unclear.
Previous studies on SII and CVD risk have several limitations. First, existing analyses have primarily assumed linear relationships, potentially overlooking complex nonlinear associations between SII and CVD outcomes. Second, there has been insufficient consideration of key lifestyle confounders, particularly comprehensive adjustment for physical activity patterns and dietary factors. Third, the integration of SII with established clinical risk prediction tools like the FRS remains unexplored in population-based studies. To address these limitations, our study specifically: (1) employs restricted cubic splines (RCS) to characterize potential non-linear relationships, (2) incorporates enhanced adjustment for objectively measured lifestyle factors including device-based physical activity and dietary intake, and (3) evaluates the relationship between SII and the FRS in a nationally representative sample. This study was based on the National Health and Nutrition Examination Survey (NHANES) to assess the association of SII with the FRS.
Materials and methods
Data and sample source
The NHANES is a cross-sectional survey conducted by the National Center for Health Statistics (NCHS) in the United States. The NCHS and the Centers for Disease Control and Prevention (CDC) conducted the survey. The NCHS’ Research Ethics Review Board evaluated and approved the NHANES study protocol. The study protocol was approved by the NCHS Ethics Review Board, and all participants provided informed consent. For further confirmation, please refer to the link to the NCHS ethics approval document for the NHANES data1. Briefly, the NHANES uses a stratified and multistage probability approach, which surveys approximately 5,000 participants annually. The data collected includes demographic data, questionnaire data, laboratory data, examination data and limited access data.
There were a total of 101,316 participants included in the NHANES from 1999 to 2018. Of those, we excluded 62,568 participants because their age was younger than 30 or older than 74; 3,676 subjects were excluded due to imponderable total cholesterol (TC); 2 individuals were excluded due to imponderable high-density lipoprotein (HDL); 17,935 were excluded due to missing glucose; 655 participants were excluded due to imponderable blood pressure; and 58 individuals were excluded due to imponderable SII. Of 1,692 participants with CVD,448 were excluded for using NSAIDs and statins; 4,381 subjects with arthritis and thyroid disease were excluded. Finally, 9901 participants were included in this study (Figure 1).
FIGURE 1
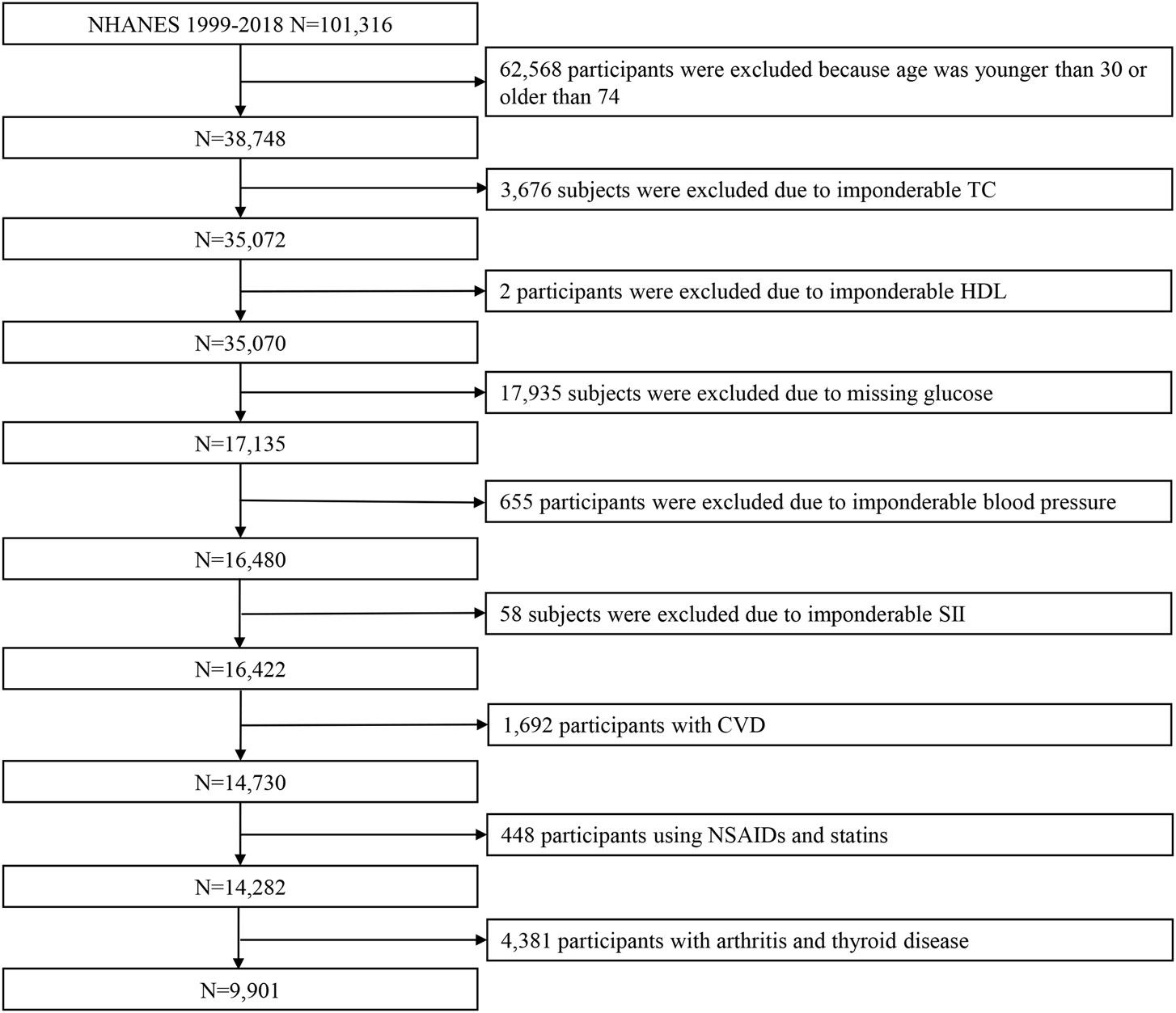
Flowchart showing the selection of participants from the NHANES from 1999 to 2018. Abbreviations: NHANES, National Health and Nutrition Examination Survey; TC, total cholesterol; HDL, high-density lipoprotein; SII, systemic immune-inflammation index; CVD, cardiovascular disease.
Systemic immune-inflammation
The SII is based on a complete blood count. Standardized protocols for measuring blood counts (platelets, lymphocytes, and neutrophils) were provided by the NHANES Laboratory Procedures Manual. More details can be found at2. In the present study, the following equation was utilized to calculate the SII (SII = platelet count × neutrophil count ⁄ lymphocyte count) [17]. The SII data were unevenly distributed and skewed to the right. Natural logarithmic transformation converts absolute differences into proportional changes. For clinical interpretation, Ln-transformed SII (Ln(SII)) values can be back-transformed to the original scale. A one-unit increase in Ln (SII) corresponds to an e-fold (≈2.72-fold) multiplicative change in the original SII values. Based on this, the SII data need to be Ln-transformed. Supplementary Figure S1 shows the distributional characteristics and normality assessment of SII values. (A) The histogram of the raw SII values showed extreme right-skewness. (B) The quantile-quantile plot confirmed severe non-normality (Anderson-Darling A = 617.20, P < 0.001). (C) The histogram of Ln (SII) showed substantial improvement, although with residual skewness. (D) The quantile-quantile plot of the transformed values demonstrated a persistent deviation from normality (A = 6.62, P < 0.001). The red lines represent theoretical normal distributions.
FRS
The FRS is a general 10-year risk estimate for CVD, which was developed using a Cox model based on the Framingham Heart Study [4]. In the present study, the FRS was calculated using sex, age, TC, HDL, systolic blood pressure (SBP), treatment for hypertension, smoking and diabetes. The FRS was Ln-transformed to reduce the effects of non-normality. To eliminate the potential contribution of neighborhood clustering by age and sex on neighborhood-level variance, the outcome variable used in the analysis was the normalized residual of the Ln-transformed FRS regressed on age and sex. Low 10-year CVD risk was defined as FRS <10%; FRS ≥10% was considered intermediate or high 10-year CVD risk.
Covariates
The baseline characteristics included demographic factors (age, sex (men or women), education (high school or below and college graduate or above), marital status (married/Living with a partner, never married and widowed/divorced/separated), poverty income ratio (PIR) (2, 2-4 and 4) and ethnicity (Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black, and Other Ethnicity), behavioral factors (smoking, alcohol consumption, physical activity (PA), and energy intake), and low-density lipoprotein (LDL). The metabolic equivalent (MET) represents the rate of oxygen uptake required to maintain the body’s basic metabolic processes while at complete rest. In line with World Health Organization (WHO) recommendations, we used MET values of 3.3 for walking, 4 for moderate activities, and 8 for vigorous activities [27]. PA was valued by total MET-minutes/week. Vigorous activity was defined as accumulating at least 3,000 MET-minutes/week, while moderate activity required a minimum of 600 MET-minutes/week. Individuals who met neither criterion were classified into the light activity category. Energy intake was estimated using the dietary intake data from the 24-h period before the interview. Body mass index (BMI) was calculated by dividing weight in kilograms by the square of height in meters. Participants were categorized into four BMI groups according to WHO criteria: underweight (BMI <18.5 kg/m2), normal weight (BMI 18.5–24.9 kg/m2), overweight (BMI 25.0–29.9 kg/m2), and obese (BMI ≥30.0 kg/m2). The diagnosis of CVD was determined by self-reported physician diagnoses obtained during interviews using a standardized questionnaire. subjects were asked, “Has a doctor or other health expert ever informed you that you have angina/congestive heart failure/coronary heart disease/heart attack (myocardial infarction)/stroke?” If the answer was “yes” to any of the above questions, the subjects were considered to have CVD.
Statistical analysis
Participants were stratified into four groups according to Ln (SII) quartiles. Continuous and categorical baseline characteristics were presented as mean ± standard deviation (SD) and number (percentage), respectively. Differences in the distribution of variables were compared using a weighted one-way analysis of variance (ANOVA) test for continuous data and a weighted chi-square test for categorical data, respectively.
The Pearson test was used to analyze the correlation between Ln (SII) and the FRS. Two models were developed to assess associations of Ln (SII) and the FRS or 10-year CVD risk level by using weighted GLM: the crude model was not adjusted; the adjusted model was adjusted for education, marital status, PIR, ethnicity, alcohol consumption, PA, energy intake, BMI, and LDL. To rigorously assess the robustness of our results, we performed a subgroup analysis to investigate potential modifying effects based on ethnicity.
Furthermore, weighted RCS were used to explore non-linear relationships between Ln (SII) and FRS or 10-year CVD risk level. Knot placement and model specification were implemented using Harrell’s rms package. Interior knots were positioned at clinically relevant percentiles of the Ln (SII) distribution following established conventions: the 10th, 50th, and 90th percentiles were used for the optimal 3-knot configuration, which was determined by comparing Akaike Information Criterion (AIC) across 3 to 6 knot models. Boundary knots were automatically anchored at the observed minimum Ln (SII)value of 2.47.
All statistical analyses were performed using R version 4.2.2, with a significance threshold of P < 0.05, and all statistical tests were two-sided.
Results
Study population characteristics
A total of 9901 subjects were included in the study, among whom 53.27% were men, and 46.73% were women. The number of participants who were judged as having an intermediate or high 10-year CVD risk was 1,438 (14.52%). The demographic characteristics of the subjects by Ln (SII) quartiles are shown in Table 1. The results suggest statistically significant differences in age, gender, marital status, ethnicity, smoking status, treatment for hypertension, BMI, PA, TC, FRS, and 10-year CVD risk level (all P < 0.05). The demographic characteristics of the subjects after weighting are shown in Supplementary Table S1.
TABLE 1
| Variables | Q1 (N = 2,476) | Q2 (N = 2,475) | Q3 (N = 2,475) | Q4 (N = 2,475) | Total (N = 9901) | P |
|---|---|---|---|---|---|---|
| Age (year) (mean ± SD) | 48.02 ± 12.10 | 47.55 ± 12.22 | 46.99 ± 11.69 | 46.82 ± 12.15 | 47.34 ± 12.05 | 0.002 |
| Gender (%) | <0.001 | |||||
| Men | 1,500 (60.58) | 1,404 (56.73) | 1,275 (51.52) | 1,095 (44.24) | 5,274 (53.27) | |
| Women | 976 (39.42) | 1,071 (43.27) | 1,200 (48.48) | 1,380 (55.76) | 4,627 (46.73) | |
| Education (%) | 0.940 | |||||
| High school or below | 1,190 (48.06) | 1,181 (47.72) | 1,170 (47.27) | 1,183 (47.80) | 4,724 (47.71) | |
| College graduate or above | 1,285 (51.90) | 1,291 (52.16) | 1,303 (52.65) | 1,291 (52.16) | 5,170 (52.22) | |
| Marital status (%) | 0.002 | |||||
| Married/Living with partner | 1710 (69.06) | 1726 (69.74) | 1714 (69.25) | 1,605 (64.85) | 6,755 (68.23) | |
| Never married | 423 (17.08) | 444 (17.94) | 431 (17.41) | 526 (21.25) | 1824 (18.42) | |
| Widowed/Divorced/Separated | 322 (13.00) | 275 (11.11) | 303 (12.24) | 315 (12.73) | 1,215 (12.27) | |
| PIR (%) | 0.569 | |||||
| <2 | 982 (39.66) | 943 (38.10) | 941 (38.02) | 958 (38.71) | 3,824 (38.62) | |
| 2–4 | 635 (25.65) | 602 (24.32) | 623 (25.17) | 636 (25.70) | 2,496 (25.21) | |
| ≥4 | 646 (26.09) | 722 (29.17) | 706 (28.53) | 673 (27.19) | 2,747 (27.74) | |
| Ethnicity (%) | <0.001 | |||||
| Mexican American | 459 (18.54) | 542 (21.9) | 528 (21.33) | 489 (19.76) | 2018 (20.38) | |
| Other Hispanic | 213 (8.60) | 242 (9.78) | 243 (9.82) | 230 (9.29) | 928 (9.37) | |
| Non-Hispanic White | 690 (27.87) | 952 (38.46) | 1,062 (42.91) | 1,178 (47.60) | 3,882 (39.21) | |
| Non-Hispanic Black | 778 (31.42) | 465 (18.79) | 403 (16.28) | 373 (15.07) | 2019 (20.39) | |
| Other Ethnicity | 336 (13.57) | 274 (11.07) | 239 (9.66) | 205 (8.28) | 1,054 (10.65) | |
| Smoke (%) | <0.001 | |||||
| No | 1,459 (58.93) | 1,427 (57.66) | 1,397 (56.44) | 1,256 (50.75) | 5,539 (55.94) | |
| Yes | 1,017 (41.07) | 1,048 (42.34) | 1,078 (43.56) | 1,219 (49.25) | 4,362 (44.06) | |
| Alcohol consumption (%) | 0.567 | |||||
| No | 1709 (69.02) | 1730 (69.90) | 1734 (70.06) | 1710 (69.09) | 6,883 (69.52) | |
| Yes | 568 (22.94) | 566 (22.87) | 574 (23.19) | 595 (24.04) | 2,303 (23.26) | |
| Treatment for hypertension (%) | 0.034 | |||||
| No | 2060 (83.20) | 2033 (82.14) | 2082 (84.12) | 2009 (81.17) | 8,184 (82.66) | |
| Yes | 416 (16.80) | 442 (17.86) | 393 (15.88) | 466 (18.83) | 1717 (17.34) | |
| T2DM (%) | 0.356 | |||||
| No | 1,232 (49.76) | 1,244 (50.26) | 1,250 (50.51) | 1,291 (52.16) | 5,017 (50.67) | |
| Yes | 1,244 (50.24) | 1,231 (49.74) | 1,225 (49.49) | 1,184 (47.84) | 4,884 (49.33) | |
| BMI (kg/m2) (mean ± SD) | 27.98 ± 5.67 | 28.28 ± 5.79 | 28.78 ± 6.19 | 29.07 ± 6.80 | 28.53 ± 6.14 | <0.001 |
| Underweight | 34 (1.37) | 36 (1.45) | 28 (1.13) | 39 (1.58) | 137 (1.38) | <0.001 |
| Normal weight | 742 (29.97) | 714 (28.85) | 677 (27.35) | 671 (27.11) | 2,804 (28.32) | |
| Overweight | 612 (24.72) | 570 (23.03) | 559 (22.59) | 526 (21.25) | 2,267 (22.90) | |
| Obese | 1,068 (43.13) | 1,147 (46.34) | 1,195 (48.28) | 1,209 (48.85) | 4,619 (46.65) | |
| Energy intake (kcal) (mean ± SD) | 2,154.30 ± 945.14 | 2,152.5 ± 860.88 | 2,137.35 ± 924.32 | 2,137.03 ± 898.14 | 2,145.27 ± 907.38 | 0.859 |
| PA (MET-minutes/week) (mean ± SD) | 4,286.55 ± 8,987.26 | 3,658.71 ± 6,222.36 | 3,848.23 ± 15,673.21 | 2,993.56 ± 8,034.72 | 3,700.21 ± 10,367.76 | 0.001 |
| Vigorous | 625 (25.24) | 673 (27.19) | 731 (29.54) | 799 (32.28) | 2,828 (28.56) | <0.001 |
| Moderate | 742 (29.97) | 750 (30.30) | 719 (29.05) | 700 (28.28) | 2,911 (29.40) | |
| Light | 666 (26.90) | 606 (24.48) | 561 (22.67) | 490 (19.80) | 2,323 (23.46) | |
| TC (mg/dL) (mean ± SD) | 198.76 ± 40.91 | 199.81 ± 40.89 | 200.95 ± 39.55 | 201.96 ± 41.91 | 200.37 ± 40.84 | 0.035 |
| HDL (mg/dL) (mean ± SD) | 53.79 ± 16.46 | 52.97 ± 16.01 | 53.18 ± 16.24 | 53.64 ± 16.24 | 53.40 ± 16.24 | 0.249 |
| LDL (mg/dL) (mean ± SD) | 119.43 ± 35.18 | 120.31 ± 35.86 | 120.32 ± 33.9 | 120.32 ± 35.24 | 120.09 ± 35.05 | 0.779 |
| SBP (mm Hg) (mean ± SD) | 123.09 ± 18 | 122.61 ± 17.71 | 122.53 ± 17.87 | 122.68 ± 18.23 | 122.73 ± 17.95 | 0.695 |
| Ln (SII) (mean ± SD) | 5.46 ± 0.32 | 5.96 ± 0.09 | 6.28 ± 0.10 | 6.78 ± 0.28 | 6.12 ± 0.53 | <0.001 |
| FRS (mean ± SD) | 5.04 ± 8.12 | 5.21 ± 7.89 | 5.29 ± 8.26 | 5.78 ± 8.61 | 5.33 ± 8.23 | 0.011 |
| Low 10-year CVD risk | 2,136 (86.27) | 2,118 (85.58) | 2,136 (86.30) | 2073 (83.76) | 8,463 (85.48) | 0.032 |
| Intermediate and high 10-year CVD risk | 340 (13.73) | 357 (14.42) | 339 (13.70) | 402 (16.24) | 1,438 (14.52) |
Demographic characteristics of subjects (n = 9901) in the NHANES 1999–2018.
Abbreviations: SD, standard deviation; PIR, poverty income ratio; SBP, systolic blood pressure; T2DM, type 2 diabetes mellitus; BMI, body mass index; PA, physical activity; MET, metabolic equivalent; TC, total cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; Ln (SII), Ln-transformed SII; SII, systemic immune-inflammation index; FRS, framingham cardiovascular risk score; CVD, cardiovascular disease.
Associations of Ln (SII) with the FRS
A Pearson correlation analysis was performed to examine the correlation between Ln (SII) and the FRS, along with other continuous variables. There was a positive relationship found between Ln (SII) and the FRS, with a corresponding correlation coefficient of 0.09 (P < 0.001). Except for energy intake and PA, there were positive relationships between the other factors and the FRS. The results of the Pearson correlation coefficient are shown in Figure 2.
FIGURE 2
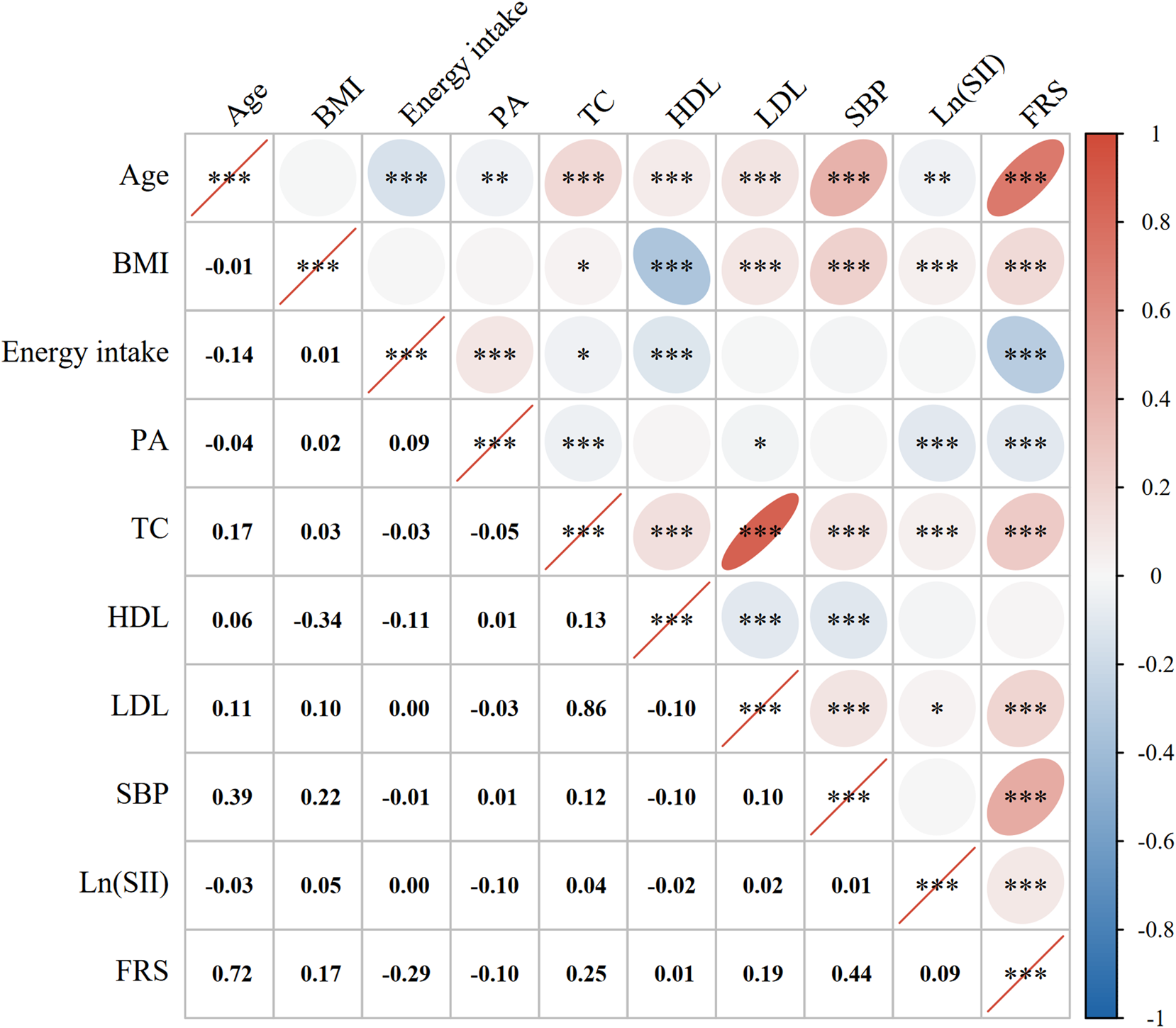
Correlation analysis. Pearson correlation coefficients between Ln (SII) with the FRS and the factors that were computed for the FRS. The correlation coefficients are shown as numbers and colors. Blue indicates a positive correlation and red indicates a negative correlation. A flatter circle represents a stronger correlation. *indicates p < 0.05; **indicates p < 0.01; ***indicates p < 0.001. Abbreviations: BMI, body mass index; PA, physical activity; TC, total cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SBP, systolic blood pressure; Ln (SII), Ln-transformed SII; SII, systemic immune-inflammation index; FRS, Framingham cardiovascular risk score.
Associations of Ln (SII) and Ln (SII) quartiles with the FRS
Table 2 suggests the results of the association between Ln (SII) and Ln (SII) quartiles and the FRS in the crude model and adjusted model. In the crude model, Ln (SII) was analyzed as a continuous variable. The odds ratio (OR) for FRS was 2.18 [95% confidence interval (CI): 1.57–3.02] per unit increment in Ln (SII). Compared to the lowest quartile (Q1) of Ln (SII), the OR (95% CI) for Q2, Q3, and Q4 were 1.22 (0.76-1.96), 1.86 (1.15–3.01), and 3.06 (1.90–4.93), respectively (P for trend <0.001). In the adjusted model, the OR (95% CI) for FRS was 1.52 (1.12–2.06) for a per-unit increment in Ln (SII). Compared to the Q1 of Ln (SII), the OR (95% CI) for Q2, Q3, and Q4 were 1.19 (0.73–1.92), 1.49 (0.95–2.35), and 1.89 (1.20–2.98), respectively (P for trend = 0.004).
TABLE 2
| Factor | Crude model | Adjusted model | ||||
|---|---|---|---|---|---|---|
| OR | 95%CI | P value | OR | 95%CI | P value | |
| Ln (SII) | 2.18 | 1.57–3.02 | <0.001 | 1.52 | 1.12–2.06 | 0.009 |
| Stratified by Ln (SII) quartiles | 1.47 | 1.26–1.71 | <0.001 | 1.24 | 1.07–1.43 | 0.004 |
| Q1 | Ref | Ref | ||||
| Q2 | 1.22 | 0.76–1.96 | 0.417 | 1.19 | 0.73–1.92 | 0.488 |
| Q3 | 1.86 | 1.15–3.01 | 0.013 | 1.49 | 0.95–2.35 | 0.086 |
| Q4 | 3.06 | 1.90–4.93 | <0.001 | 1.89 | 1.20–2.98 | 0.007 |
Associations of Ln (SII) and Ln (SII) quartiles with the FRS, as estimated by weighted generalized linear models.
The crude model was not adjusted;
The adjusted model was adjusted for education, marital status, PIR, ethnicity, alcohol consumption, PA, energy intake, BMI, and LDL.
Abbreviations: Ln (SII), Ln-transformed SII; SII, systemic immune-inflammation index; FRS, framingham cardiovascular risk score; OR, odds ratio; Ref, reference; PIR, poverty income ratio; PA, physical activity; BMI, body mass index; LDL, low-density lipoprotein.
The non-linear association between Ln (SII) and the FRS
A non-linear relationship was explored between Ln (SII) and the FRS by RCS. Figure 3 shows that a linear association was found between Ln (SII) and the FRS (P for non-linearity = 0.972). As Ln (SII) increased, so did the value of FRS (P for overall trend <0.001).
FIGURE 3
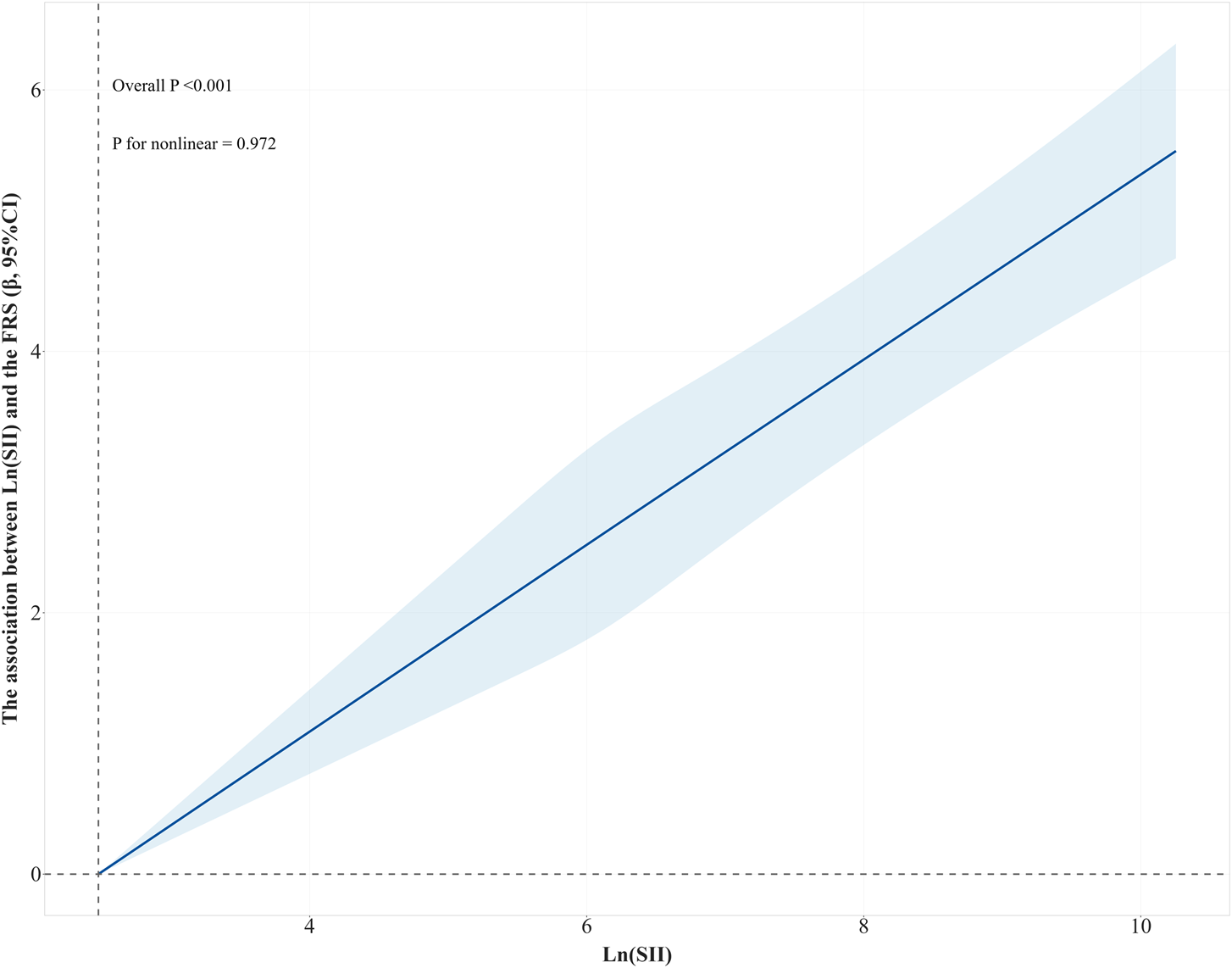
RCS curve of the association between Ln(SII) and the FRS. The results were adjusted for education, marital status, PIR, ethnicity, alcohol consumption, PA, energy intake, BMI, and LDL. Abbreviations: RCS, restricted cubic splines; Ln (SII), Ln-transformed SII; SII, systemic immune-inflammation index; FRS, Framingham cardiovascular risk score; PIR, poverty income ratio; PA, physical activity; BMI, body mass index; LDL, low-density lipoprotein.
The relationship between Ln (SII) and 10-year CVD risk
Supplementary Table S2 shows the results of the association between Ln (SII) and Ln (SII) quartiles and 10-year CVD risk in the crude model and adjusted model. After adjusting for the potential confounders, all associations became non-significant (OR range: 1.03–1.13, P > 0.05 for all comparisons). Supplementary Figure S2 shows a linear association between Ln (SII) and 10-year CVD risk (P for non-linearity = 0.541). With the Ln (SII) increasing, 10-year CVD risk gradually increased (P for overall trend <0.001).
Subgroup analysis
Supplementary Tables S3-S12 present ethnicity-stratified analyses of the association between Ln (SII) (as both continuous and quartile variables) and the FRS and 10-year CVD risk level. After fully adjusting for education, marital status, PIR, alcohol consumption, physical activity, energy intake, BMI, and LDL, we observed distinct ethnicity-associated patterns: No significant associations were found among Mexican American patients (OR range: 0.84–1.75, all P > 0.05), Other Hispanic subjects (OR range: 0.34-1.39, all P > 0.05), or Non-Hispanic Black subjects (OR range: 0.98–2.34, all P > 0.05). In the Non-Hispanic White group, higher Ln (SII) was associated with an increased FRS (per-unit OR = 1.72, 95% CI: 1.13–2.60; Q4 vs. Q1 OR = 2.30, 95% CI: 1.27–4.16; P for trend = 0.007), but it was not associated with 10-year CVD risk (OR range: 1.05–1.18). Conversely, the Other Ethnicity group exhibited an inverse 10-year CVD risk association (per-unit OR = 0.46, 95% CI: 0.27–0.79; Q3 vs. Q1 OR = 0.41, 95% CI: 0.19–0.90), whereas no significant association was observed for the FRS (OR range: 0.48–0.86).
Discussion
It is increasingly recognized that systemic inflammation initiates and exacerbates the pathological processes of chronic diseases. Many inflammatory predictors associated with CVD risk have been identified [28]. The present study showed that the FRS increased with the increase of SII. Both the Pearson correlation analysis and RCS revealed a significant positive association between Ln (SII) and the FRS. Therefore, SII may serve as a useful biomarker for assessing 10-year CVD risk in the general population. SII could be used to quickly identify high-risk subjects with a 10-year risk of CVD at a relatively low cost.
Several studies have supported the finding of a positive relationship between SII and the CVD risk [24, 25, 29]. Another study suggested that an elevation of SII level would be obvious in almost all subtypes of CVD, including ischemic stroke, hemorrhagic stroke, myocardial infarction, and peripheral arterial disease [30]. Furthermore, SII was found to be associated with poor short-term prognosis in atrial fibrillation patients with ischemic stroke [31]. Meanwhile, SII levels were also elevated in patients with ST-segment elevation myocardial infarction [32, 33]. Based on these, a close relationship was found to exist between SII and CVD risk. These results also provide sufficient evidence that chronic inflammation in the healthy population greatly increases the risk of developing CVD.
The underlying mechanisms of SII relating to the FRS can be attributed to several factors. First, chronic systemic inflammation can cause abnormal platelet aggregation, allowing them to adhere to the surface of endothelial cells, causing hypoxia, ischemia and microthrombus formation, leading to local tissue death [34, 35]. Second, long-term aberrant decrease of lymphocyte counts indicates excessive lymphocyte death in the human body, leading to reduced immune system capacity and immune dysfunction. Subsequently, lymphocyte death could further lead to endothelial dysfunction, abnormal aggregation of platelets, and thrombosis after platelet activation [24]. Third, monocytes and neutrophils can also promote abnormal coronary plaque status by activating and generating inflammatory responses, inducing atherosclerotic plaque rupture and thrombosis, thereby increasing the risk of adverse cardiovascular events [36]. As a complex inflammatory index, SII could effectively and comprehensively reflect the inflammatory state and immune system state of the body. The cumulative effect of the interaction between the three different cell lines synergistically enhances the association between systemic inflammation and the 10-year CVD risk assessed by the FRS. Therefore, the interaction between platelets, neutrophils, and lymphocytes may represent a potential therapeutic target for chronic inflammation in patients with high FRS.
Subgroup analyses revealed complex heterogeneity in the relationship between Ln (SII) and the FRS: significant positive associations were observed in Non-Hispanic Whites, whereas null effects were found in the Mexican American, Other Hispanic, and Non-Hispanic Black groups. In contrast, the Other Ethnicity group exhibited a negative relationship between Ln (SII) and 10-year CVD risk. These differential patterns likely reflect the interplay between ethnicity, SII and CVD. The findings in the Mexican American/Hispanic/Other Ethnicity groups may reflect the “health paradox” phenomenon [37, 38]. The reason for the different relationships between different ethnic groups likely originates from three interconnected mechanisms: First, racism generates population health disparities through the propagation of beliefs, attitudes, and treatment of group members by both individuals and institutions [39]. Second, some research suggests that Black individuals in the United States experience premature death from a variety of causes, including multiple diseases [40, 41]. Second, the immigrant health advantage has always played a critical role in this situation. Several studies have shown lower mortality rates [42], and fewer chronic conditions [43] in immigrants than in US-born subjects. Third, current ethnic classifications inadequately capture heterogeneity, while undifferentiated pan-ethnic labels mask critical variations in nativity and phenotype [44].
It is important to emphasize the limitations of the present study. First, the NHANES had a cross-sectional design, which limits its ability to establish causality. Specifically, simultaneous SII and FRS measurements prevent determining whether inflammation precedes or results from subclinical CVD; influencing factors (e.g., occult infections, undiagnosed autoimmune disorders) may independently influence both SII and CVD risk. Furthermore, single-time-point in front sampling cannot capture how the relationship between SII and CVD risk evolves over time. Considering the large sample size of the NHANES and its complex, multi-stage, probabilistic sampling design, the results still indicated stability in the relationship between SII and 10-year CVD risk. Second, due to missing information in the data set, excluded participants with incomplete data may introduce bias into the analysis. Third, hematological parameters were assessed at a single time point (a limitation inherent to the NHANES protocol), while the substantial sample size provides sufficient statistical power to detect meaningful population-level associations. Large-scale epidemiological investigations have consistently demonstrated that single measurements of inflammatory biomarkers retain significant predictive value for long-term cardiovascular risk stratification in adult populations. In addition, utilizing the NHANES database inevitably introduces the possibility of imprecise data capture and recall bias.
Conclusion
The results demonstrate a significant positive association between SII and the FRS, supporting the potential of SII as an effective biomarker for identifying 10-year CVD risk. Nonetheless, variations in the relationship between SII and the FRS among different ethnic groups underscore the importance of careful application. Further studies with larger and more diverse cohorts are required for comprehensive validation.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The studies involving humans were approved by the National Center for Health Statistics’ Research Ethics Assessment Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
All authors participated in the design, interpretation of the study and analysis of the data and review of the manuscript. YY designed this study. RT, LZ, GX, and TY analyzed the data. YY, QL, and CW wrote the manuscript, and HY reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors thank all NHANES staff and participants for their contributions, including data collection and sharing.
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.ebm-journal.org/articles/10.3389/ebm.2025.10704/full#supplementary-material
Footnotes
1.^www.cdc.gov/nchs/nhanes/irba98.htm
2.^https://www.cdc.gov/nchs/nhanes/biospecimens/serum_plasma_urine.htm
References
1.
AhmadFBAndersonRN. The leading causes of death in the US for 2020. Jama (2021) 325:1829–30. 10.1001/jama.2021.5469
2.
TsaoCWAdayAWAlmarzooqZIAndersonCAMAroraPAveryCLet alHeart disease and stroke statistics-2023 update: a report from the American heart association. Circulation (2023) 147:e93–e621. 10.1161/cir.0000000000001123
3.
NdumeleCENeelandIJTuttleKRChowSLMathewROKhanSSet alA synopsis of the evidence for the science and clinical management of cardiovascular-kidney-metabolic (CKM) syndrome: a scientific statement from the American heart association. Circulation (2023) 148:1636–64. 10.1161/CIR.0000000000001186
4.
D’AgostinoRBSrVasanRSPencinaMJWolfPACobainMMassaroJMet alGeneral cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation (2008) 117:743–53. 10.1161/circulationaha.107.699579
5.
NyirendaM. Assessment of cardiovascular disease risks using Framingham risk scores (FRS) in HIV-positive and HIV-negative older adults in South Africa. Prev Med Rep (2021) 22:101352. 10.1016/j.pmedr.2021.101352
6.
GaleCRCooperCSayerAA. Framingham cardiovascular disease risk scores and incident frailty: the English longitudinal study of ageing. Age (Dordrecht, Netherlands) (2014) 36:9692. 10.1007/s11357-014-9692-6
7.
KwobahEKoenNMwangiAAtwoliLSteinDJ. Prevalence of lifestyle cardiovascular risk factors and estimated framingham 10-year risk scores of adults with psychotic disorders compared to controls at a referral hospital in Eldoret, Kenya. BMC psychiatry (2023) 23:909. 10.1186/s12888-023-05409-0
8.
BirhanuMMEvansRGZenginARiddellMKalyanramKKartikKet alAbsolute cardiovascular risk scores and medication use in rural India: a cross-sectional study. BMJ open (2022) 12:e054617. 10.1136/bmjopen-2021-054617
9.
AdamoLRocha-ResendeCPrabhuSDMannDL. Reappraising the role of inflammation in heart failure. Nat Rev Cardiol (2020) 17:269–85. 10.1038/s41569-019-0315-x
10.
KaptogeSSeshasaiSRGaoPFreitagDFButterworthASBorglykkeAet alInflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur Heart J (2014) 35:578–89. 10.1093/eurheartj/eht367
11.
RupareliaNChaiJTFisherEAChoudhuryRP. Inflammatory processes in cardiovascular disease: a route to targeted therapies. Nat Rev Cardiol (2017) 14:133–44. 10.1038/nrcardio.2016.185
12.
ZhengHYinZLuoXZhouYZhangFGuoZ. Associations between systemic immunity-inflammation index and heart failure: evidence from the NHANES 1999-2018. Int J Cardiol (2024) 395:131400. 10.1016/j.ijcard.2023.131400
13.
YoshiharaF. Systemic inflammation is a key factor for mortality risk stratification in chronic kidney disease patients with coronary Artery Calcification. Circ J (2016) 80:1537–8. 10.1253/circj.CJ-16-0506
14.
LiXCuiLXuH. Association between systemic inflammation response index and chronic kidney disease: a population-based study. Front Endocrinol (2024) 15:1329256. 10.3389/fendo.2024.1329256
15.
Carrillo-SalinasFJNgwenyamaNAnastasiouMKaurKAlcaideP. Heart inflammation: immune cell roles and Roads to the heart. The Am J Pathol (2019) 189:1482–94. 10.1016/j.ajpath.2019.04.009
16.
StrassheimDDempseyECGerasimovskayaEStenmarkKKaroorV. Role of inflammatory cell subtypes in heart failure. J Immunol Res (2019) 2019:1–9. 10.1155/2019/2164017
17.
HuBYangXRXuYSunYFSunCGuoWet alSystemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res (2014) 20:6212–22. 10.1158/1078-0432.Ccr-14-0442
18.
TabasIGarcía-CardeñaGOwensGK. Recent insights into the cellular biology of atherosclerosis. The J Cel Biol (2015) 209:13–22. 10.1083/jcb.201412052
19.
TiwariRLSinghVBarthwalMK. Macrophages: an elusive yet emerging therapeutic target of atherosclerosis. Med Res Rev (2008) 28:483–544. 10.1002/med.20118
20.
LibbyP. Current concepts of the pathogenesis of the acute coronary syndromes. Circulation (2001) 104:365–72. 10.1161/01.cir.104.3.365
21.
TanYHuBLiQCaoW. Prognostic value and clinicopathological significance of pre-and post-treatment systemic immune-inflammation index in colorectal cancer patients: a meta-analysis. World J Surg Oncol (2025) 23:11. 10.1186/s12957-025-03662-z
22.
WangBLTianLGaoXHMaXLWuJZhangCYet alDynamic change of the systemic immune inflammation index predicts the prognosis of patients with hepatocellular carcinoma after curative resection. Clin Chem Lab Med (Cclm) (2016) 54:1963–9. 10.1515/cclm-2015-1191
23.
DongMShiYYangJZhouQLianYWangDet alPrognostic and clinicopathological significance of systemic immune-inflammation index in colorectal cancer: a meta-analysis. Ther Adv Med Oncol (2020) 12:1758835920937425. 10.1177/1758835920937425
24.
XiaYXiaCWuLLiZLiHZhangJ. Systemic immune inflammation index (SII), system inflammation response index (SIRI) and risk of all-cause mortality and cardiovascular mortality: a 20-year Follow-Up cohort study of 42,875 US adults. J Clin Med (2023) 12:1128. 10.3390/jcm12031128
25.
JinZWuQChenSGaoJLiXZhangXet alThe associations of two novel inflammation Indexes, SII and SIRI with the risks for cardiovascular diseases and all-cause mortality: a ten-year follow-up study in 85,154 individuals. J Inflamm Res (2021) 14:131–40. 10.2147/jir.S283835
26.
LiJHeDYuJChenSWuQChengZet alDynamic status of SII and SIRI Alters the risk of cardiovascular diseases: evidence from Kailuan cohort study. J Inflamm Res (2022) 15:5945–57. 10.2147/jir.S378309
27.
DiaoXLingYZengYWuYGuoCJinYet alPhysical activity and cancer risk: a dose-response analysis for the Global Burden of Disease Study 2019. Cancer Commun (London, England) (2023) 43:1229–43. 10.1002/cac2.12488
28.
AzambujaMI. Inflammation as the cause of coronary heart disease. The Lancet Infect Dis (2010) 10:142–3. 10.1016/s1473-3099(10)70029-3
29.
XuMChenRLiuLLiuXHouJLiaoJet alSystemic immune-inflammation index and incident cardiovascular diseases among middle-aged and elderly Chinese adults: the Dongfeng-Tongji cohort study. Atherosclerosis (2021) 323:20–9. 10.1016/j.atherosclerosis.2021.02.012
30.
YeZHuTWangJXiaoRLiaoXLiuMet alSystemic immune-inflammation index as a potential biomarker of cardiovascular diseases: a systematic review and meta-analysis. Front Cardiovasc Med (2022) 9:933913. 10.3389/fcvm.2022.933913
31.
LinKBFanFHCaiMQYuYFuCLDingLYet alSystemic immune inflammation index and system inflammation response index are potential biomarkers of atrial fibrillation among the patients presenting with ischemic stroke. Eur J Med Res (2022) 27:106. 10.1186/s40001-022-00733-9
32.
EsenboğaKKurtulAYamantürkYYTanTSTutarDE. Systemic immune-inflammation index predicts no-reflow phenomenon after primary percutaneous coronary intervention. Acta Cardiol (2022) 77:59–65. 10.1080/00015385.2021.1884786
33.
HuangJZhangQWangRJiHChenYQuanXet alSystemic immune-inflammatory index predicts clinical outcomes for elderly patients with acute myocardial infarction receiving percutaneous coronary intervention. Med Sci Monitor (2019) 25:9690–701. 10.12659/msm.919802
34.
BankaALGuevaraMVBrannonERNguyenNQSongSCadyGet alCargo-free particles divert neutrophil-platelet aggregates to reduce thromboinflammation. Nat Commun (2023) 14:2462. 10.1038/s41467-023-37990-z
35.
von Ungern-SternbergSNIVogelSWalker-AllgaierBGeueSMaurerAWildAMet alExtracellular Cyclophilin A Augments platelet-Dependent thrombosis and thromboinflammation. Thromb Haemost (2017) 117:2063–78. 10.1160/th17-01-0067
36.
AnkenyRFHindsMTNeremRM. Dynamic shear stress regulation of inflammatory and thrombotic pathways in baboon endothelial outgrowth cells. Tissue Eng A (2013) 19:1573–82. 10.1089/ten.TEA.2012.0300
37.
AriasEEschbachKSchaumanWSBacklundELSorliePD. The Hispanic mortality advantage and ethnic misclassification on US death certificates. Am J Public Health (2010) 100(Suppl. 1):S171–7. 10.2105/ajph.2008.135863
38.
BoenCEHummerRA. Longer-but harder-lives? The hispanic health paradox and the social determinants of racial, ethnic, and immigrant-native health disparities from midlife through late life. J Health Soc Behav (2019) 60:434–52. 10.1177/0022146519884538
39.
GeeGCWalsemannKMBrondoloE. A life course perspective on how racism may be related to health inequities. Am J Public Health (2012) 102:967–74. 10.2105/ajph.2012.300666
40.
Dwyer-LindgrenLKendrickPKellyYOSylteDOSchmidtCBlackerBFet alLife expectancy by county, race, and ethnicity in the USA, 2000-19: a systematic analysis of health disparities. The Lancet (2022) 400:25–38. 10.1016/s0140-6736(22)00876-5
41.
GuptaRAgrawalRBukhariZJabbarAWangDDiksJet alHigher comorbidities and early death in hospitalized African-American patients with Covid-19. BMC Infect Dis (2021) 21:78. 10.1186/s12879-021-05782-9
42.
DupreMEGuDVaupelJW. Survival differences among native-born and foreign-born older adults in the United States. PloS one (2012) 7:e37177. 10.1371/journal.pone.0037177
43.
GormanBKReadJGKruegerPM. Gender, acculturation, and health among Mexican Americans. J Health Soc Behav (2010) 51:440–57. 10.1177/0022146510386792
44.
ChinnJJHummerRA. Racial disparities in Functional limitations among Hispanic women in the United States. Res Aging (2016) 38:399–423. 10.1177/0164027515620244
Summary
Keywords
systemic immune inflammation index, cardiovascular disease, framingham cardiovascular risk scores, 10-year cardiovascular disease risk, inflammation
Citation
Yang Y, Tu R, Zhu L, Xu G, Yang T, Li Q, Wang C and Yang H (2025) Association between systemic immune-inflammation index and 10-year risk of cardiovascular disease in the United States (NHANES 1999–2018). Exp. Biol. Med. 250:10704. doi: 10.3389/ebm.2025.10704
Received
10 June 2025
Accepted
28 July 2025
Published
21 August 2025
Volume
250 - 2025
Updates
Copyright
© 2025 Yang, Tu, Zhu, Xu, Yang, Li, Wang and Yang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Honghui Yang, yanghonghui@zzu.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.