Abstract
Peripheral nerve injuries (PNIs) pose a significant clinical challenge, often leading to incomplete functional recovery despite current treatments. Platelet-rich plasma (PRP), which contains high levels of growth factors and bioactive molecules, has emerged as a promising regenerative therapy for nerve repair and restoring function. This review consolidates current evidence on PRP applications in treating peripheral nerve injuries, examining molecular mechanisms, clinical outcomes, and therapeutic potential. PRP markedly enhances nerve regeneration, improves recovery of sensory and motor functions, and alleviates neuropathic pain across various nerve injuries. It promotes axonal growth, reduces scar formation, stimulates Schwann cell proliferation, and modulates inflammation through the release of neurotrophic factors, including PDGF, VEGF, TGF-β, and IGF-1. Combining PRP with surgical techniques and biomaterial scaffolds yields better therapeutic results. Key factors influencing efficacy include platelet concentration, leukocyte content, activation methods, and patient-specific variables. PRP is a safe and effective option for peripheral nerve injury repair. However, challenges persist in standardizing preparation protocols, optimizing treatment timing, and fully understanding molecular mechanisms. Future research should focus on personalized PRP formulations, combination therapies, and large-scale randomized controlled trials to develop definitive clinical guidelines.
Impact statement
Peripheral nerve injuries often lead to long-term disability, and current treatment options offer limited functional recovery. This review is important because it consolidates and critically evaluates the growing body of research on the use of platelet-rich plasma (PRP) as a novel, biologically based therapy for peripheral nerve repair. While PRP has gained attention in various fields of regenerative medicine, its role in nerve healing is still emerging and not yet standardized. By bringing together recent findings from both preclinical and clinical studies, this work provides new insight into how PRP promotes nerve regeneration through anti-inflammatory effects, stimulation of nerve-supporting cells, and delivery of growth factors that accelerate healing. It also explores how PRP can be combined with existing surgical and biomaterial approaches for improved outcomes. This review contributes to the field by highlighting both the therapeutic promise and the current limitations of PRP, and by outlining future research directions needed to optimize its clinical application. As such, it helps define a clearer path forward for integrating PRP into routine nerve injury management.
Introduction
The central nervous system (CNS), comprising the brain and spinal cord, acts as the central control hub that communicates with various body organs via an extensive network of nerve fibres extending throughout the peripheral nervous system. This communication occurs through electrical and chemical signals that facilitate coordinated physiological functions and responses to environmental stimuli. These peripheral nerves can be systematically classified based on their anatomical locations and functional characteristics into three primary categories: mixed nerves (containing both sensory and motor fibres), motor nerves (responsible for muscle contraction and movement), and sensory nerves (transmitting sensory information from receptors to the CNS) [1, 2].
Peripheral nerve injury (PNI) represents a significant global health concern and a leading cause of long-term disability, affecting millions of individuals worldwide with substantial socioeconomic implications. The consequences of PNI are often devastating, resulting in severe sensory-motor dysfunction that impairs daily activities, chronic neurogenic pain that significantly reduces quality of life, and potential permanent disability requiring long-term rehabilitation [3–5]. The etiology of PNI is diverse and multifactorial, encompassing neurodegenerative diseases that progressively damage nerve structure and function, acute open trauma from accidents or surgical procedures, and chronic nerve compression syndromes such as carpal tunnel syndrome or cubital tunnel syndrome [6, 7].
In the anatomically complex head and neck region, peripheral nerve injuries pose challenges due to the critical functional roles of affected nerves. Damage commonly affects several key cranial and peripheral nerves, including the inferior alveolar nerve (resulting in altered sensation in the lower lip and chin), the lingual nerve (causing taste disturbances and tongue numbness), the facial nerve (leading to facial paralysis and expression difficulties), and the hypoglossal nerve (affecting tongue movement and speech articulation). These injuries can severely impact essential functions such as mastication, speech, facial expression, and overall oral function [8].
While peripheral nerve fibres demonstrate a remarkable regenerative capacity and can achieve spontaneous healing within weeks to months under optimal conditions, this natural recovery process is often incomplete or insufficient, especially in cases involving significant nerve damage, large gaps, or unfavourable local conditions [9]. The clinical reality presents considerable therapeutic challenges, as fewer than half of patients with documented PNI undergo surgical nerve repair, often due to factors such as delayed diagnosis, patient comorbidities, or lack of specialized surgical expertise. Among those who do receive surgical intervention, only 40–50% attain complete functional recovery, highlighting the limitations of current treatment approaches and the urgent need for improved options [10]. Current management strategies include both surgical techniques (such as direct repair, nerve grafting, and nerve transfers) and conservative methods, with non-surgical treatments encompassing targeted physiotherapy programmes, emerging cell-based therapies using stem cells or Schwann cells (SC), and pharmaceutical interventions aimed at managing pain and encouraging nerve regeneration [3–5, 11].
Platelet-rich plasma (PRP) represents an innovative autologous biological therapeutic derived through centrifugal separation of the patient’s own blood, specifically isolating the plasma fraction enriched with platelet concentrations that typically exceed normal physiological levels by 3-5-fold [12, 13]. This preparation process involves collecting whole blood, followed by specific centrifugation protocols that concentrate platelets while preserving their functional integrity and bioactive properties. The resulting PRP product serves as a potent reservoir of endogenous bioactive molecules, being particularly rich in multiple growth factors and cytokines essential for tissue repair and regeneration. These include granulocyte-macrophage colony-stimulating factor (GM-CSF) for cellular proliferation, vascular endothelial growth factor A (VEGF-A) for angiogenesis, epithelial growth factor (EGF) for cellular differentiation, transforming growth factor β (TGF-β) for tissue remodeling, platelet-derived growth factor (PDGF) for cellular migration and proliferation, hepatocyte growth factor (HGF) for neuroprotection, and insulin-like growth factor 1 (IGF-1) for nerve regeneration [14].
PRP has established a substantial clinical track record demonstrating therapeutic efficacy across diverse medical applications, including accelerated healing in sports-related injuries, enhanced recovery in spinal cord trauma, improved wound healing in chronic conditions, and successful outcomes in plastic and reconstructive surgery procedures [15]. Specifically in the context of peripheral nerve injuries, PRP exhibits multifaceted therapeutic mechanisms, demonstrating significant neurogenic properties that promote nerve fibre regeneration, neuroprotective effects that prevent secondary nerve degeneration, and anti-inflammatory activities that modulate detrimental neuroinflammation while creating a favourable microenvironment for healing [8, 16–18]. These comprehensive therapeutic effects are mediated through PRP’s complex role in orchestrating nerve regeneration processes, including SC proliferation, axonal sprouting, remyelination, and its documented capacity to alleviate debilitating neuropathic pain through modulation of inflammatory pathways and pain signaling mechanisms [19]. Compelling clinical evidence continues to emerge supporting PRP’s therapeutic potential, as exemplified by the case study conducted by García de Cortázar et al. [20], who documented satisfactory neurological recovery and functional improvement in a patient with significant nerve injury following a structured PRP treatment protocol administered over 11 months [20].
Given the demonstrated therapeutic potential of PRP in managing peripheral nerve injuries, along with the urgent clinical need for more effective treatment methods to improve functional recovery, this comprehensive review aims to systematically summarize, critically analyze, and discuss current research progress on PRP applications for PNI. The review will evaluate both preclinical and clinical evidence, treatment protocols, outcomes, and future research directions to enhance PRP-based therapies for peripheral nerve injury management.
Application of PRP in the treatment of peripheral nerve injury
Accumulating evidence from both preclinical and clinical studies shows that PRP has multiple therapeutic properties vital for peripheral nerve repair. The main reason for PRP’s effectiveness is its ability to regulate neuroinflammation through a dual mechanism involving direct platelet-derived anti-inflammatory mediators and the recruitment of reparative cell populations that release additional anti-inflammatory factors [21–23]. When activated, platelets in PRP release stored anti-inflammatory cytokines such as interleukin-10 (IL-10) and TGF-β, while also attracting macrophages, mesenchymal stem cells, and other regenerative cells to the injury site. These recruited cells further enhance the anti-inflammatory environment by secreting more anti-inflammatory mediators, resulting in a sustained therapeutic effect that lasts beyond the initial platelet activation phase.
Beyond these anti-inflammatory properties, PRP exhibits significant neuroprotective capabilities by preventing secondary neuronal death and promoting axonal survival following peripheral nerve injury. Furthermore, the neurogenic properties of PRP are mediated through the release of neurotrophic factors, including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and neurotrophin-3 (NT-3), which stimulate axonal sprouting, guide nerve fiber growth, and support the maintenance of neuronal phenotype during the regeneration process [1, 24]. Consequently, these growth factors work synergistically to create an optimal microenvironment that facilitates both proximal nerve stump survival and distal target reinnervation.
Building upon these fundamental mechanisms, the therapeutic potential of PRP has been extensively investigated across various peripheral nerve injuries, with consistent positive outcomes reported for multiple anatomical locations. Comprehensive studies have documented PRP’s efficacy in treating injuries to major peripheral nerves, including the sciatic nerve, facial nerve, median nerve, and even applications extending to central nervous system pathologies [1, 21–24]. Moreover, the versatility of PRP treatment is further evidenced by its successful application in diverse clinical scenarios, ranging from complete nerve transection repairs to functional restoration across peripheral nerve gaps [25].
In addition to its regenerative capabilities, PRP therapy has demonstrated significant analgesic properties in treating neuropathic pain associated with peripheral nerve injuries. The pain-relieving mechanisms involve the downregulation of pro-inflammatory cytokines and the modulation of pain signaling pathways at both peripheral and central levels [18, 25–27]. Notably, recent studies have shown that PRP application effectively reduces neuropathic pain in osteoarthritis patients by specifically downregulating microglial activation in the spinal cord, thereby interrupting the central sensitization processes that contribute to chronic pain states [28]. This dual peripheral and central mechanism of pain relief represents a significant advantage over conventional analgesic approaches.
The mechanisms underlying these regenerative effects are significantly mediated through PRP’s impact on Schwann cell biology. SC play a crucial role in peripheral nerve regeneration by providing structural support, producing neurotrophic factors, and facilitating remyelination of regenerating axons. Supporting this understanding, Salarinia et al. [29] demonstrated that PRP treatment significantly enhances SC proliferation in experimental spinal cord injury models in rats, leading to improved functional outcomes. Subsequently, investigations have confirmed PRP’s ability to promote both SC migration to injury sites and their subsequent proliferation, creating a cellular environment conducive to nerve repair [25, 30]. These cellular effects are attributed to the growth factors present in PRP, particularly PDGF and HGF, which specifically target SC receptors and activate proliferation pathways.
Collectively, the diverse applications and mechanisms of PRP in peripheral nerve regeneration have been systematically documented across multiple research studies, with findings consistently supporting its therapeutic value in nerve repair, functional restoration, pain management, and treatment of degenerative neurological conditions [8, 31–36]. The comprehensive body of evidence regarding PRP applications in various peripheral nerve regeneration scenarios is summarized in Figure 1, providing a systematic overview of treatment protocols, outcomes, and clinical effectiveness across different nerve injury types and anatomical locations.
FIGURE 1
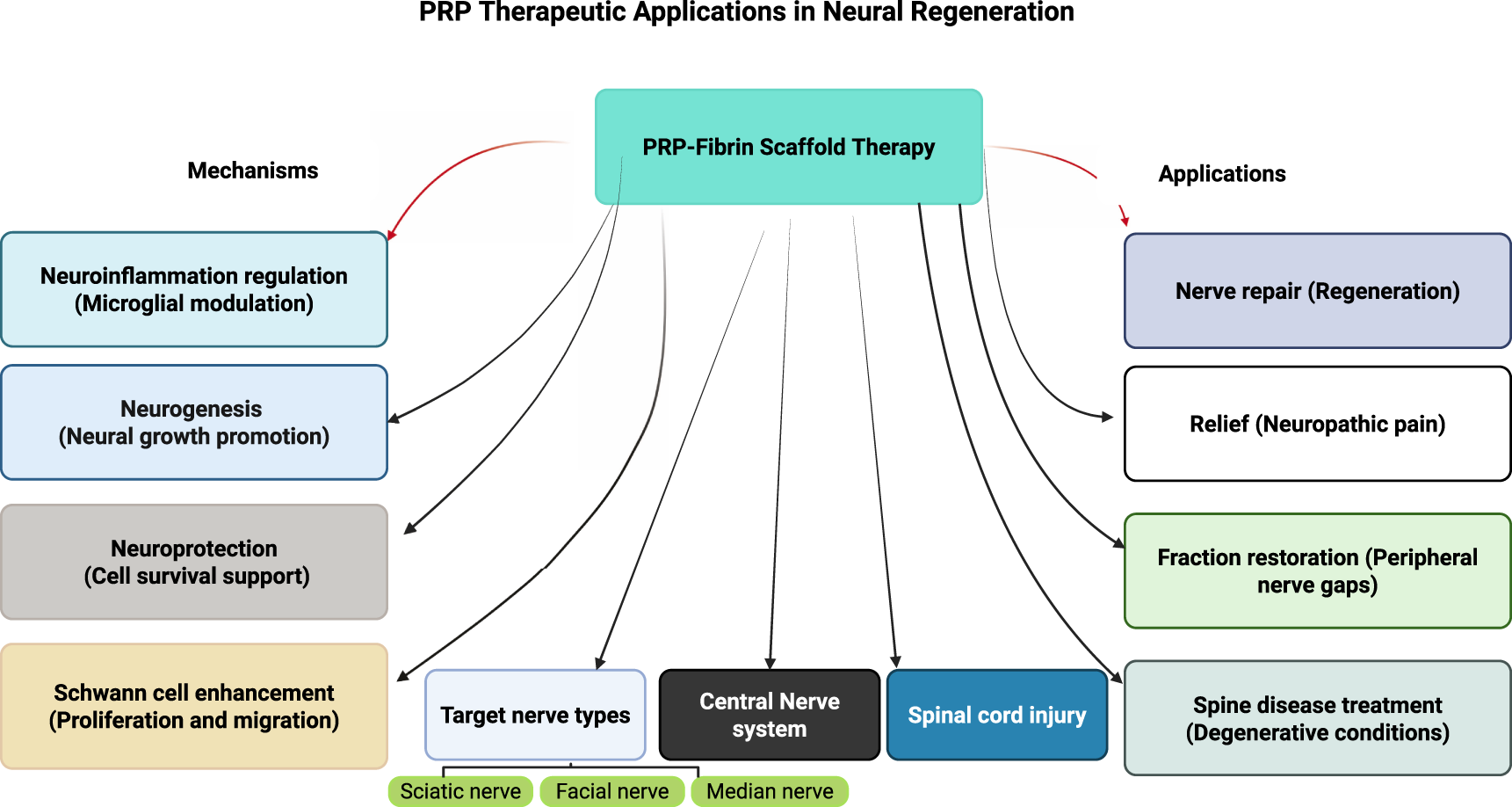
Comprehensive framework of platelet-rich plasma therapeutic applications in neural regeneration.
Cellular and molecular mechanisms driving PRP therapy effects
The process of concentrating and separating platelets from the patient’s own blood is known as PRP therapy. Upon activation, these platelets release a potent array of bioactive molecules stored within their granules, including cytokines, growth factors, and signaling molecules. These components are fundamental orchestrators of tissue repair and wound healing processes [37]. A primary mechanism involves the direct stimulation of cellular proliferation and differentiation essential for regeneration. Growth factors within PRP, such as PDGF, TGF-β, and VEGF, activate key cell types: mesenchymal stem cells (MSCs), promoting their proliferation and differentiation into various tissue-specific lineages; endothelial cells, stimulating angiogenesis to improve local vascularization; and fibroblasts, enhancing their synthesis of crucial extracellular matrix (ECM) components like collagen and fibronectin [38, 39].
Furthermore, PRP actively promotes the production of essential structural molecules, including fibronectin, collagen, and hyaluronic acid. Collectively, these molecules form a provisional ECM scaffold. This scaffold provides critical mechanical support to the healing site, facilitates cell migration, and creates a conducive environment for tissue regeneration [40]. Critically, PRP also activates and recruits local endogenous stem cells within the injury site, amplifying their potential to differentiate into the specific cell types needed for functional tissue repair [41]. Crucially, the efficacy of these regenerative processes, cellular activation, differentiation, and ECM synthesis, is profoundly enhanced by PRP’s ability to modulate the inflammatory environment, shifting it towards a state optimal for repair [42, 43]. PRP exerts significant immunomodulatory actions, suppressing detrimental chronic inflammation and actively promoting resolution and regeneration. This anti-inflammatory activity is intrinsically linked to creating the permissive conditions necessary for the cellular and matrix-building activities described previously.
PRP achieves this essential immunomodulation through several interconnected pathways. Firstly, it serves as a rich source of potent anti-inflammatory molecules, including interleukin-1 receptor antagonist (IL-1ra), Interleukin-4 (IL-4), and Interleukin-10 (IL-10). IL-1ra directly inhibits the potent pro-inflammatory cytokine IL-1β, while IL-4 and IL-10 suppress the production of other key pro-inflammatory mediators like IL-6 and TNF-α, simultaneously promoting anti-inflammatory signaling cascades [42, 44]. Secondly, PRP stimulates the polarization of macrophages away from the pro-inflammatory M1 phenotype towards the anti-inflammatory, pro-repair M2 phenotype. These M2 macrophages secrete high levels of TGF-β and IL-10, further dampening inflammation, exhibit enhanced phagocytic activity to clear cellular debris, and directly contribute to tissue remodeling [44]. Thirdly, components within PRP, notably TGF-β and Prostaglandin E2 (PGE2), act to regulate T-cell responses. TGF-β induces cell cycle arrest and apoptosis in T-cells, while PGE2 downregulates essential co-stimulatory molecules and cytokine receptors on their surface, thereby inhibiting T-cell activation and proliferation [45]. Fourthly, PRP influences dendritic cell (DC) function, promoting the development of tolerogenic DCs. These specialized DCs exhibit reduced expression of pro-inflammatory cytokines and co-stimulatory molecules, instead fostering immune tolerance and the generation of regulatory T-cells (Tregs) [46]. Finally, PRP directly enhances the development, proliferation, and function of Tregs themselves. Tregs are essential for maintaining immune tolerance; they suppress effector T-cells and other immune cells through mechanisms involving the release of anti-inflammatory cytokines (IL-10, TGF-β) and direct cell contact [47].
By orchestrating this complex immunomodulation alongside its direct regenerative effects on cells and matrix synthesis, PRP comprehensively supports the sequential phases of wound healing. During the initial inflammatory phase, PRP helps resolve inflammation efficiently and promotes the formation of granulation tissue. Subsequently, it actively drives the proliferation and migration of key cells like fibroblasts, endothelial cells, and keratinocytes. Finally, it supports the remodeling phase by providing the necessary matrix components and signals. This integrated action accelerates the overall healing process and enhances the functional quality and structural integrity of the regenerated tissue [48]. A schematic overview of PRP’s cellular and molecular mechanisms is presented in Figure 2.
FIGURE 2
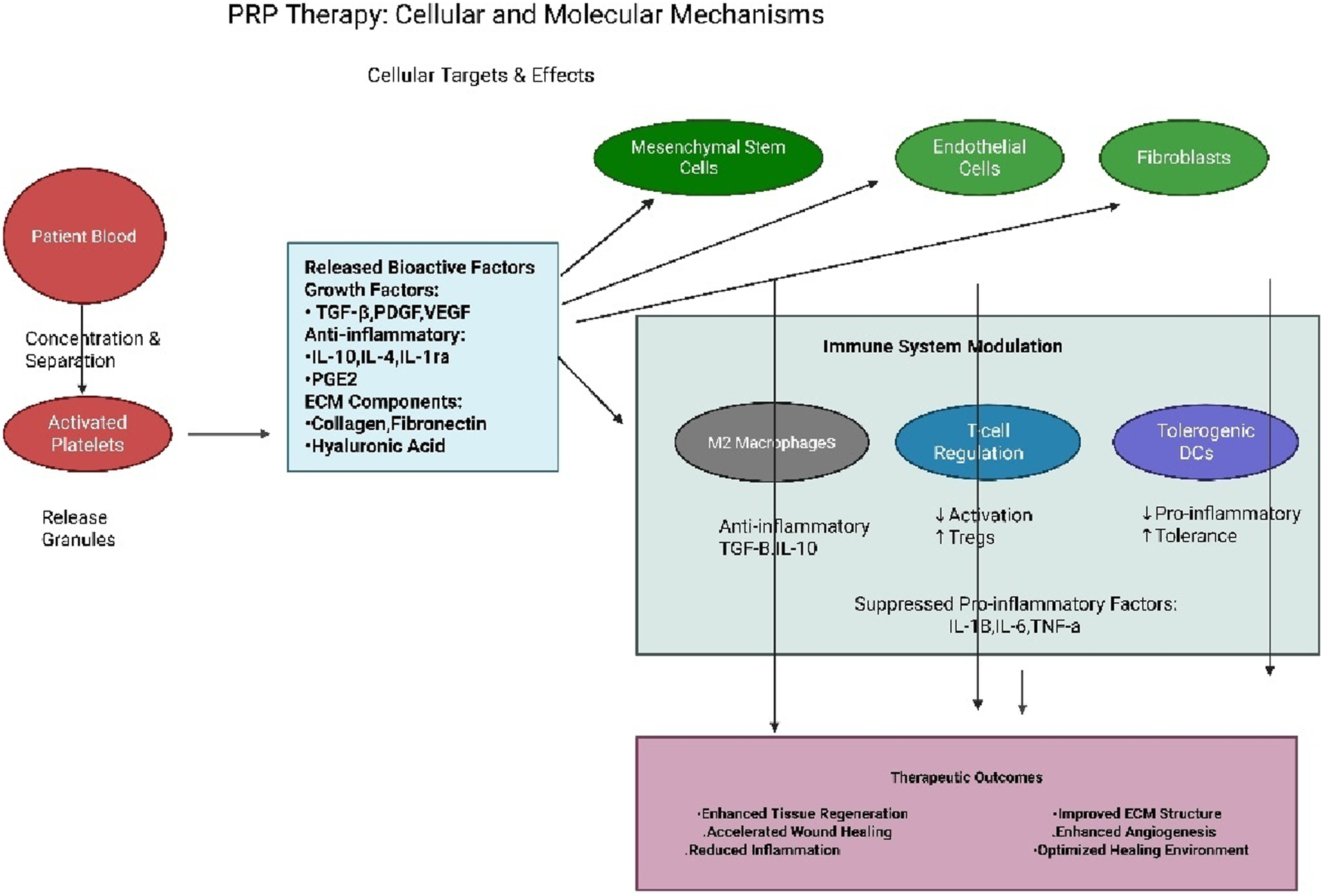
The schematic presentation of PRP cellular and molecular therapy.
The combination of surgical and rehabilitative techniques with PRP therapy
The combination of surgical and rehabilitative techniques with PRP therapy encompasses multiple temporal approaches, each serving distinct therapeutic purposes. A recent exploratory study in rabbits demonstrated that preoperative PRP treatment of the implantation site significantly enhanced fat graft survival, with decreased inflammation and fibrosis and markedly improved angiogenesis compared to control groups [49].
Building upon this preoperative foundation, the therapeutic potential of PRP can be further maximized through its strategic application during the surgical procedure itself. Intraoperative PRP application refers to the planned incorporation of PRP as an integral component of the surgical procedure. This approach is typically applied during the final stages of surgery rather than throughout the entire operation, representing a primary therapeutic goal rather than an ancillary treatment. Surgeons strategically apply platelet-rich plasma directly to the surgical site or incorporate it into biological scaffolds immediately before wound closure. This optimizes tissue integration and regeneration through growth factors and bioactive substances that stimulate neovascularization and extracellular matrix production, ensuring maximum therapeutic benefit while the surgical site remains accessible [50].
Postoperative PRP therapy involves administering PRP injections into the surgical site during the recovery period to accelerate tissue regeneration and enhance functional recovery. This approach differs from intraoperative application as it occurs days to weeks after the initial surgical procedure. Postoperative PRP significantly accelerates tissue regeneration, reduces post-surgical inflammation, and promotes optimal wound healing during the critical recovery phase. The therapy directly delivers growth factors and cytokines to the surgical site through targeted injections that promote angiogenesis, collagen deposition, and cellular proliferation [51].
Integration with rehabilitation protocols represents an advanced approach where PRP therapy is strategically combined with physical therapy and rehabilitation programs following surgical procedures. Physical therapists can incorporate PRP injections as a complementary tool within comprehensive rehabilitation programs to enhance neuromuscular re-education, improve joint stability, and accelerate tissue repair processes. This integrated approach leverages PRP’s ability to enhance the healing of injured tissues, reduce pain levels, and improve muscle strength. The synergistic effect of combining biological enhancement with mechanical stimulation ultimately enables a more complete and expedited return to pre-injury functional capacity [52].
Impact of PRP in peripheral nerve injury recovery
The role of PRP in peripheral nerve injury recovery has been summarized in Table 1. PRP represents a concentrated autologous preparation derived from patient blood that contains elevated concentrations of platelets and bioactive growth factors essential for tissue regeneration. The therapeutic mechanism underlying PRP’s efficacy centers on its ability to secrete critical growth factors, including PDGF, which promotes cellular proliferation; TGF-β, which modulates inflammatory responses and stimulates tissue repair; and VEGF, which promotes neovascularization to support regenerating neural tissue. Given that PRP is autologous in nature, it exhibits minimal risk of immunogenic reactions, establishing it as a promising therapeutic modality for peripheral nerve injury management [76].
TABLE 1
| PRP treatment | Outcomes in PNI | Species | References |
|---|---|---|---|
| PRP | PRP enhances locomotor recovery, spares white matter, promotes angiogenesis and neuronal regeneration, and modulates blood vessel size, leading to the recovery of spinal cord injuries | Rat | [15] |
| PRP | Promoted the radial nerve in musculoskeletal disorders | Human | [20] |
| PRP-derived exosomes | Promoted SC proliferation and dorsal root ganglion axon growth Increased the ability of MSCs to promote neural repair and regeneration in patients with PNI. |
Mouse | [23, 24] |
| PRP gel +Collagen film | Promoted facial nerve regeneration | Rats | [53] |
| PRP | Recovered facial nerve injury followed by promoting vibrissae movement, eyelid closure, and electrophysiological function | Rat | [54] |
| PRP gel +Collagen/Chitosan composite film | Promoted the proliferation of SC, nerve regeneration and functional recovery in rats with sciatic nerve injury | Rat | [55] |
| PRP+ Low-dose ultrashort wave therapy | Improve sciatic nerve crush injury regeneration and recovery | Rabbit | [30] |
| PRP | Promoted nerve regeneration through improvement of angiogenesis and intracellular ubiquitin levels by regulating ITGB8, ribosomal protein S27a (RSP27a) and ubiquilin 1 (UBQLN1) | Rabbit | [56] |
| PRP + gelatin methacrylate and sodium alginate hydrogel | Enhances growth factors VEGF-A and PDGF-followed by promotion of the migration of SC and the neovascularization of endothelial cells to prevent sciatic nerve defects and facilitate the repairing of peripheral nerve | Rat | [57] |
| Platelet-rich fibrin | The axon regeneration of the sciatic nerve and sensory function was improved with nerve conduit filled with platelet-rich fibrin Repair peripheral nerve defects | Mouse | [58] |
| PRP-derived exosomes | Promoted the nerve regeneration by enhancing the proliferation, migration, and secretion of trophic factors by SC | Rat | [59] |
| PRP | Enhanced the proliferation, secretion and migration of SCs and the regeneration of axons in the early stage as well as VEGF expression and improved repairing of tibial nerve defects | Rabbit | [60] |
| Freeze-dried PRP | Increased the expression of nerve growth factor and S100B Induced neuro-regeneration and relieved chronic orofacial pain |
Rats | [61] |
| PRP | Treatment of recurrent laryngeal nerve injury Regeneration Promoted the proliferation and migration of SC |
Rabbit | [62] |
| Human umbilical cord blood+ PRP | Improved the spinal cord injury regeneration | Rat | [63, 64] |
| PRP + chitin | Facilitate the repairing of sciatic nerve defects | Rat | [65] |
| Platelet-rich fibrin | Enhanced the sciatic nerve regeneration | Rat | [66] |
| PRP | Enhanced the mature SC proliferation, and microenvironment in the small gap and promote peripheral nerve regeneration | Rabbit | [67] |
| PRP+ adipose tissue–derived stem cells | Improve recovering of sciatic nerve repairing and prevent its defects | Rat | [68] |
| PRP+ adipose tissue–derived stem cells | Enhanced the spinal cord injury recovery and improved of repairing central nervous system | Rat | [69] |
| PRP + Citicoline | Improved sciatic nerve injury following recovery of peripheral nerve injury | Rat | [70] |
| Leukocyte-platelet rich fibrin | Suppressed proinflammatory cytokines followed by prevention of peripheral nerve inflammation and injuries Facilitated peripheral nerve regeneration |
Rat | [71] |
| PRP | Induces nerve regeneration by promoting neurotrophic factors and anti-inflammatory cytokines by calcium chloride activation Facilitated the recovery of spinal cord dorsal root repair |
Rat | [71] |
| PRP | Improved regeneration and proliferation of SC | Rabbit | [72] |
| Platelet-rich fibrin | Facilitated the regeneration of sciatic nerves and peripheral nerve injury | Rat | [73] |
| Platelet-rich fibrin | Improved the regeneration of sciatic nerve Showed positive effect on maxillofacial tissues regeneration |
Rabbit | [74] |
| PRP | Promoted the healing of digital nerve crush injury Decreased the neuropathic pain |
Human | [75] |
Therapeutic potential of PRP in peripheral nerve injury treatment.
Numerous preclinical and clinical studies across various nerve types, including facial, sciatic, and median nerves, have widely supported the positive effect of PRP on peripheral nerve healing [30, 53, 55–57, 77–85]. In models of facial nerve injury, experimental research showed that PRP can greatly enhance therapeutic outcomes when combined with biocompatible materials like chitosan, which serves as a structured scaffold for controlled and sustained release of growth factors at the injury site [53, 78]. Li et al. showed that PRP has neuroprotective effects on traumatic facial nerve injuries, with notable recovery of SC and significant axonal regeneration [54]. Likewise, studies on sciatic nerve injury consistently indicate that autologous PRP supports nerve regeneration by decreasing M1 macrophages and altering the inflammatory environment [32, 79, 80, 86–89].
PRP has been shown to stimulate SC proliferation and secretion while promoting angiogenesis and affecting intracellular signaling pathways. Notably, PRP significantly upregulated the expression of integrin subunit β-8 (ITGB8), which plays a critical role in angiogenesis after nerve injury [56]. When combined with biomaterial scaffolds such as collagen/chitosan composite membranes or gelatin methacrylate hydrogels, PRP has demonstrated enhanced efficacy in promoting both functional and structural nerve recovery [55, 81, 82].
In median nerve applications, particularly for carpal tunnel syndrome treatment, PRP has shown superior outcomes compared to corticosteroids in clinical trials, providing significant pain relief and functional improvement [77, 83–85, 90–93]. Studies have demonstrated that ultrasound guided PRP injections can provide effective treatment for up to 1 year post-injection, with predictive factors including patient body weight, distal motor latency, and median nerve cross-sectional area [77, 85].
In recent years, progress in platelet research has highlighted the significance of platelet-derived extracellular vesicles (EVs), including exosomes, and their role in facilitating intercellular communication [94]. A study conducted by Yi et al. [59] isolated platelet-rich plasma–derived exosomes (PRP-Exos) and found that they markedly promoted SC proliferation, migration, and secretion of trophic factors. Additionally, PRP-Exos induced notable changes in both transcriptional and protein expression within SCs, especially increasing the expression of genes crucial for nerve repair. In a rat sciatic nerve crush model, the application of ultrasound-targeted microbubble destruction (UTMD) significantly improved the delivery of PRP-Exos to the injury site, resulting in greater exosome accumulation locally and enhanced regenerative and functional outcomes compared to untreated controls [59].
Other studies have shown that PRP-Exos improve MSC survival by reducing apoptosis, preserving stemness, and delaying senescence. Pretreated MSCs (pExo-MSCs) demonstrated better retention in vivo, resulting in enhanced axonal regeneration, remyelination, and neurological recovery. In vitro, they further encouraged SC proliferation and dorsal root ganglion axonal extension, mainly through glial cell–derived neurotrophic factor (GDNF) secretion and activation of the PI3K/Akt pathway [24].
Similarly, Zhang et al. (2024) reported that PRP-Exo–treated MSCs (MSC^PExo) enhanced SC proliferation and reduced apoptosis after peripheral nerve injury (PNI). Conditioned medium from MSCPExo (MSCPExo-CM) further stimulated SC proliferation, migration, and angiogenesis. Proteomic analysis of the MSCPExo secretome identified 440 proteins, many of which showed strong pro-regenerative and angiogenic functions. ELISA confirmed the enrichment of key trophic factors, and Western blotting validated PI3K/Akt pathway activation. Collectively, these findings highlight PRP-Exos as potent enhancers of MSC paracrine activity and valuable modulators of neural repair [23].
Factors affecting PRP therapy in nerve repair
The factors affecting PRP effectiveness are detailed in Table 2 and depicted graphically in Figure 3 for enhanced clarity. The technique used for preparation, the parameters of centrifugation, and patient-specific characteristics such as age and health condition can significantly influence the composition of PRP. Research demonstrates that PRP efficacy decreases with increasing age, with PRP derived from young donors (18–35 years) showing significantly better therapeutic outcomes compared to PRP from older donors (≥65 years) [124]. Studies show that growth factor levels, including PDGF-BB, TGF-β1, IGF-1, and EGF, are statistically higher in subjects younger than 25 years compared to those aged 26 years or older [125]. Additionally, PRP derived from women older than 45 years does not contain significantly higher concentrations of bioactive components compared to younger groups, suggesting that aging significantly affects the active components of PRP [126]. At the cellular level, elderly patients show decreased numbers of α-granules in platelets, which are the main component releasing active substances, leading to decreased platelet function [127]. Clinical evidence supports these laboratory findings, with PRP therapy showing poor efficacy in elderly patients (≥60 years) for conditions such as facial rejuvenation and Achilles tendinitis treatment [124].
TABLE 2
| Parameters affecting PRP efficacy | Biological outcomes of PRP | References |
|---|---|---|
| Concentration of platelet | The platelets concentration in PRP can vary depending on how it is prepared, and the equipment used. Higher platelet concentrations are generally associated with better outcomes, but there is an optimal range, and too high concentrations may not be beneficial | [95, 96] |
| Contents of leukocyte | PRP can be categorized as either leukocyte-rich or leukocyte-poor, depending on whether leukocytes are present or absent. The amount of leukocytes present can impact the inflammatory response and the healing of tissues | [97–100] |
| Method of activation for PRP | PRP can be activated through different methods, including thrombin, calcium chloride, or exposure to collagen. This activation subsequently triggers the release of growth factors from platelets, thereby influencing the regenerative properties of PRP. | [101–104] |
| Buffy coat removal | The method used to separate the buffy coat from whole blood during PRP preparation determines the purity and composition of PRP. | [105, 106] |
| Time and speed utilized for centrifugation | The separation of blood components and the final composition of PRP are determined by the speed and duration of centrifugation. It is crucial to use optimal centrifugation parameters to obtain PRP with the desired properties | [107–109] |
| Types of anticoagulants | Anticoagulants like citrate or heparin are utilized to prevent clotting while collecting blood. The selection of anticoagulant can impact the activation of platelets and the stability of PRP. | [43, 106, 110] |
| Injectable formulation | PRP can be administered in either liquid or gel form, depending on the specific clinical application. The injectable form chosen has a significant impact on the ease of administration and how PRP is distributed within the tissues | [111–114] |
| Composition of growth factor | The concentration and composition of growth factors, such as TGF-β, PDGF, and VEGF, can vary among different PRP preparations. The specific growth factors released by the platelets and their concentrations play a critical role in the regenerative and healing processes | [115–117] |
| Injected PRP volume | The distribution, diffusion, and therapeutic effects of PRP in the target tissue can be influenced by the volume injected | [70, 107] |
| Factors specific to the patient | The response to PRP treatment can be affected by various factors, including age, sex, underlying health conditions, and medications | [118–120] |
| Clinical hallmarks | The choice of PRP preparation and administration protocol is influenced by the specific condition being treated, such as tendonitis, osteoarthritis, or wound healing, as well as the targeted tissue PRP therapy may be more effective for certain types of tissues, such as tendons, ligaments, and cartilage, compared to others. Additionally, mild to moderate injuries tend to respond better to PRP than severe or chronic conditions. Furthermore, areas with a good blood supply may exhibit enhanced healing with the use of PRP therapy |
[18, 34, 121] |
| Content of fibrin | Fibrin, present in PRP, plays a crucial role in both clot formation and tissue healing. Certain classification systems differentiate PRP preparations based on their fibrin content, categorizing them as either fibrin-rich or fibrin-poor, according to their clotting characteristics and regenerative capabilities | [43, 122] |
| Contamination of red blood cells | Contamination of red blood cells (RBCs) in PRP can significantly impact the quality and efficacy of PRP in various therapeutic applications | [41, 123] |
Factors affecting the efficacy of PRP.
FIGURE 3
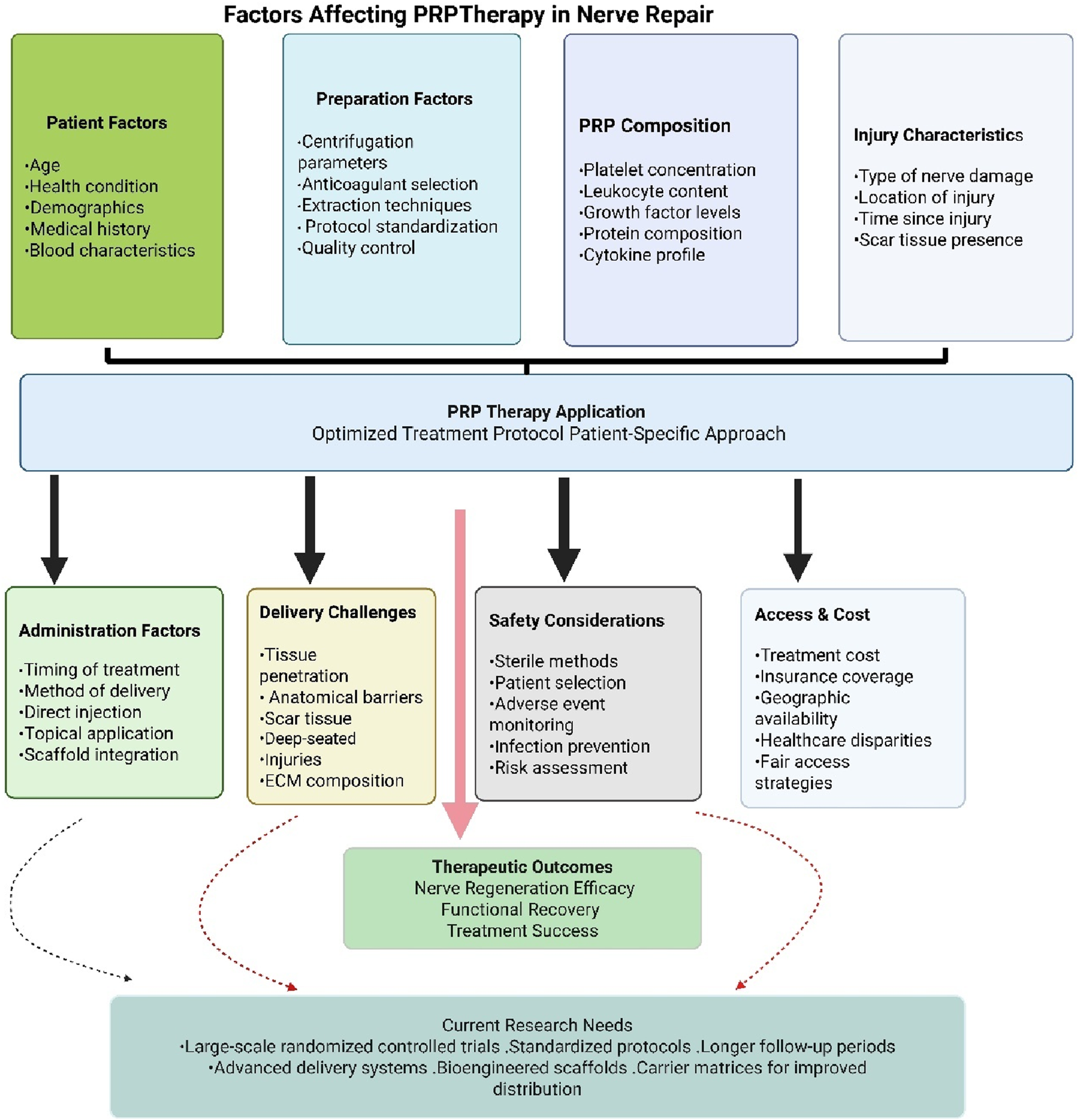
Summary of factors affecting PRP therapeutic potential.
The therapeutic efficacy of PRP in peripheral nerve repair demonstrates significant variability due to differences in leukocyte content, growth factor concentrations, and platelet density across various PRP formulations [128–130]. This heterogeneity underscores the critical need for standardized preparation protocols encompassing appropriate anticoagulant selection, optimal centrifugation parameters, and consistent extraction methodologies to ensure reproducible outcomes in both research and clinical applications [131, 132]. Despite encouraging results from preclinical investigations and early-phase clinical trials, the current body of clinical evidence remains insufficient to definitively establish PRP’s effectiveness in nerve repair and regeneration. Existing studies frequently exhibit methodological limitations, including inadequate sample sizes, absence of appropriate control groups, heterogeneous patient populations, and insufficient follow-up durations. Large-scale randomized controlled trials employing standardized protocols and extended observation periods are essential to establish the safety profile, therapeutic efficacy, and optimal clinical applications of PRP therapy in nerve regeneration [133]. Achieving adequate PRP distribution and tissue penetration presents additional complexities, particularly in cases involving scar tissue formation or deep-seated injuries. Anatomical barriers, tissue density variations, and extracellular matrix composition may impede PRP penetration into the neuronal microenvironment, potentially limiting regenerative efficacy. Advanced delivery systems incorporating carrier matrices or bioengineered scaffolds may enhance PRP distribution and retention at nerve injury sites [88]. While PRP therapy generally demonstrates a favorable safety profile, specific risks associated with nerve regeneration applications include hypersensitivity reactions, iatrogenic nerve injury, hematoma formation, and infection. Rigorous adherence to sterile protocols, careful patient selection criteria, and comprehensive adverse event monitoring are essential to minimize these risks and ensure treatment safety [134]. Additionally, therapeutic accessibility remains constrained by economic factors, particularly in regions where insurance coverage or healthcare systems do not support the costs of PRP therapy. Geographic and institutional limitations further compound healthcare disparities. Addressing these challenges requires comprehensive strategies to reduce treatment costs, expand reimbursement coverage, and improve therapeutic accessibility to ensure equitable patient access to this potentially beneficial regenerative approach [110].
Conclusion
The exploration of PRP as a treatment target for PNIs has shown significant promise, especially when PRP is obtained through plasmapheresis. This review emphasizes the positive outcomes seen in both clinical and preclinical studies, where PRP treatment has been linked to better nerve regeneration, improved sensory and motor functions, and less pain. Preclinical studies have provided valuable insights into how PRP promotes nerve repair, including encouraging axonal growth and reducing scar formation. Despite these promising results, several obstacles remain when turning preclinical findings into clinical practice. These include species-specific differences and the need for thorough clinical evaluations to confirm safety and effectiveness in humans. Standardizing PRP preparation methods and optimizing treatment timing are essential steps to improve the consistency and reliability of PRP therapy outcomes. Future research should aim to better understand the molecular mechanisms behind PRP’s therapeutic effects, refine treatment protocols, and expand its clinical use. By tackling these challenges and integrating insights from both human and animal studies, the full potential of PRP as a strong option for nerve regeneration and functional recovery in patients with PNI can be achieved.
Statements
Author contributions
Conceptualization, methodology, writing – original draft, data curation, validation, writing – review and editing, KS, YL, and AQ; supervision, resources, and funding: KS. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Tai’an Science and Technology Development Plan Project (2019NS236).
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
References
1.
Wang S Liu Z Wang J Cheng L Hu J Tang J . Platelet-rich plasma (PRP) in nerve repair. Regenerative Ther (2024) 27:244–50. 10.1016/j.reth.2024.03.017
2.
Wei C Guo Y Ci Z Li M Zhang Y Zhou Y . Advances of schwann cells in peripheral nerve regeneration: from mechanism to cell therapy. Biomed and Pharmacother (2024) 175:116645. 10.1016/j.biopha.2024.116645
3.
Zhang PX Li C Liu SY Pi W . Cortical plasticity and nerve regeneration after peripheral nerve injury. Neural Regen Res (2021) 16:1518–23. 10.4103/1673-5374.303008
4.
Xu JG Xing XX Zheng MX Hua XY Ma SJ Ma ZZ . Brain plasticity after peripheral nerve injury treatment with massage therapy based on resting-state functional magnetic resonance imaging. Neural Regen Res (2021) 16:388–93. 10.4103/1673-5374.290912
5.
Martínez‐Marcos A Sañudo JR . Cranial nerves: morphology and clinical relevance. The Anatomical Rec (2019) 302:555–7. 10.1002/ar.24106
6.
Gu D Xia Y Ding Z Qian J Gu X Bai H et al Inflammation in the peripheral nervous system after injury. Biomedicines (2024) 12:1256. 10.3390/biomedicines12061256
7.
Tian H Chen BP Li R Qu WR Zhu Z Liu J et al Interaction between schwann cells and other cells during repair of peripheral nerve injury. Neural Regen Res (2021) 16:93–8. 10.4103/1673-5374.286956
8.
Wang S Liu X Wang Y . Evaluation of platelet-rich plasma therapy for peripheral nerve regeneration: a critical review of literature. Front Bioeng Biotechnol (2022) 10:808248. 10.3389/fbioe.2022.808248
9.
Sammartino G Tia M Marenzi G Espedito di Lauro A D’Agostino E Claudio PP . Use of autologous platelet-rich plasma (PRP) in periodontal defect treatment after extraction of impacted mandibular third molars. J Oral Maxillofacial Surg (2005) 63:766–70. 10.1016/j.joms.2005.02.010
10.
Kouyoumdjian JA Graça C Ferreira VM . Peripheral nerve injuries: a retrospective survey of 1124 cases. Neurol India (2017) 65:551–5. 10.4103/neuroindia.ni_987_16
11.
He F Gu XS Chu XL Song XZ Li Q Li YR et al Basic mechanisms of peripheral nerve injury and treatment via electrical stimulation. Neural Regen Res (2022) 17:2185–93. 10.4103/1673-5374.335823
12.
Machado ES Leite R Dos Santos CC Artuso GL Gluszczak F de Jesus LG et al Turn Down - turn up: a simple and low-cost protocol for preparing platelet-rich plasma. Clinics (Sao Paulo) (2019) 74:e1132. 10.6061/clinics/2019/e1132
13.
Mercader-Ruiz J Beitia M Delgado D Sánchez P Porras B Gimeno I et al Current challenges in the development of platelet-rich plasma-based therapies. Biomed Res Int (2024) 2024:6444120. 10.1155/2024/6444120
14.
Malange KF de Souza DM Lemes JBP Fagundes CC Oliveira ALL Pagliusi MO et al The implications of brain-derived neurotrophic factor in the biological activities of platelet-rich plasma. Inflammation (2024) 48:426–46. 10.1007/s10753-024-02072-9
15.
Chen NF Sung CS Wen ZH Chen CH Feng CW Hung HC et al Therapeutic effect of platelet-rich plasma in rat spinal cord injuries. Front Neurosci (2018) 12:252. 10.3389/fnins.2018.00252
16.
Pandunugrahadi M Irianto KA Sindrawati O . The optimal timing of platelet-rich plasma (PRP) injection for nerve lesion recovery: a preliminary study. Int J Biomater (2022) 2022:1–7. 10.1155/2022/9601547
17.
Cao Y Zhu X Zhou R He Y Wu Z Chen Y . A narrative review of the research progress and clinical application of platelet-rich plasma. Ann Palliat Med (2021) 10:4823–9. 10.21037/apm-20-2223
18.
Huang S Li Q Li X Ye H Zhang L Zhu X . Recent research progress of wound healing biomaterials containing platelet-rich plasma. Int J Nanomedicine (2025) 20:3961–76. 10.2147/ijn.s506677
19.
Bohren Y Timbolschi DI Muller A Barrot M Yalcin I Salvat E . Platelet-rich plasma and cytokines in neuropathic pain: a narrative review and a clinical perspective. Eur J Pain (2022) 26:43–60. 10.1002/ejp.1846
20.
García de Cortázar U Padilla S Lobato E Delgado D Sánchez M . Intraneural platelet-rich plasma injections for the treatment of radial nerve section: a case report. J Clin Med (2018) 7:13. 10.3390/jcm7020013
21.
Jin X Fu J Lv R Hao X Wang S Sun M et al Efficacy and safety of platelet-rich plasma for acute nonarteritic anterior ischemic optic neuropathy: a prospective cohort study. Front Med (Lausanne) (2024) 11:1344107. 10.3389/fmed.2024.1344107
22.
Smail SW Abdulqadir SZ Alalem LSS Rasheed TK Khudhur ZO Mzury AFA et al Enhancing sciatic nerve regeneration with osteopontin-loaded acellular nerve allografts in rats: effects on macrophage polarization. Tissue and Cell (2024) 88:102379. 10.1016/j.tice.2024.102379
23.
Zhang Y Yi D Hong Q Liu C Chi K Liu J et al Platelet-rich plasma-derived exosomes enhance mesenchymal stem cell paracrine function and nerve regeneration potential. Biochem Biophysical Res Commun (2024) 699:149496. 10.1016/j.bbrc.2024.149496
24.
Zhang Y Yi D Hong Q Cao J Geng X Liu J et al Platelet-rich plasma-derived exosomes boost mesenchymal stem cells to promote peripheral nerve regeneration. J Controlled Release (2024) 367:265–82. 10.1016/j.jconrel.2024.01.043
25.
Kuffler DP . PRP and other techniques for restoring function across peripheral nerve gaps. J Neurorestoratology (2024) 12:100131. 10.1016/j.jnrt.2024.100131
26.
Kataria S Wijaya JH Patel U Yabut K Turjman T Ayub MA et al The role of platelet rich plasma in vertebrogenic and discogenic pain: a systematic review and meta-analysis. Curr Pain Headache Rep (2024) 28:825–33. 10.1007/s11916-024-01274-y
27.
Schol J Tamagawa S Volleman TNE Ishijima M Sakai D . A comprehensive review of cell transplantation and platelet-rich plasma therapy for the treatment of disc degeneration-related back and neck pain: a systematic evidence-based analysis. JOR Spine (2024) 7:e1348. 10.1002/jsp2.1348
28.
Yan X Ye Y Wang L Xue J Shen N Li T . Platelet-rich plasma alleviates neuropathic pain in osteoarthritis by downregulating microglial activation. BMC Musculoskelet Disord (2024) 25:331. 10.1186/s12891-024-07437-7
29.
Salarinia R Sadeghnia HR Alamdari DH Hoseini SJ Mafinezhad A Hosseini M . Platelet rich plasma: effective treatment for repairing of spinal cord injury in rat. Acta Orthopaedica et Traumatologica Turcica (2017) 51:254–7. 10.1016/j.aott.2017.02.009
30.
Zhu Y Jin Z Wang J Chen S Hu Y Ren L et al Ultrasound-guided platelet-rich plasma injection and multimodality ultrasound examination of peripheral nerve crush injury. NPJ Regen Med (2020) 5:21. 10.1038/s41536-020-00101-3
31.
Apostolakis S Kapetanakis S . Platelet-rich plasma for degenerative spine disease: a brief overview. Spine Surg Relat Res (2024) 8:10–21. 10.22603/ssrr.2023-0079
32.
Bastami F Vares P Khojasteh A . Healing effects of platelet-rich plasma on peripheral nerve injuries. J Craniofac Surg (2017) 28:e49–e57. 10.1097/scs.0000000000003198
33.
Fernandes M Valente S Santos J Furukawa R Fernandes C Leite V et al Platelet-rich fibrin conduits as an alternative to nerve autografts for peripheral nerve repair. J Reconstr Microsurg (2017) 33:549–56. 10.1055/s-0037-1603355
34.
Delgado D Padilla S Sánchez M Garate A . Platelet-rich plasma, an adjuvant biological therapy to assist peripheral nerve repair. Neural Regen Res (2017) 12:47–52. 10.4103/1673-5374.198973
35.
Spartalis E Athanasiou A Spartalis M Moris D Papalampros A Nikiteas N . Platelet-rich plasma and peripheral nerve regeneration: a potential contraindication to its use after tumor excision. Expert Opin Biol Ther (2017) 17:1045–6. 10.1080/14712598.2017.1349480
36.
Dou X-Y An M . Advances in the application of platelet-rich plasma in peripheral nerve injuries. Anesthesiology Perioper Sci (2025) 3:19. 10.1007/s44254-025-00100-x
37.
Anitua E Pino A Azkargorta M Elortza F Prado R . High-throughput proteomic analysis of human dermal fibroblast response to different blood derivatives: autologous topical serum derived from plasma rich in growth factors (PRGF) versus Leukocyte- and platelet-rich plasma (L-PRP). Biomolecules (2022) 12:1002. 10.3390/biom12071002
38.
Tatsis D Vasalou V Kotidis E Anestiadou E Grivas I Cheva A et al The combined use of platelet-rich plasma and adipose-derived mesenchymal stem cells promotes healing. A review of experimental models and future perspectives. Biomolecules (2021) 11:1403. 10.3390/biom11101403
39.
Foster TE Puskas BL Mandelbaum BR Gerhardt MB Rodeo SA . Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med (2009) 37:2259–72. 10.1177/0363546509349921
40.
Stoffels JM Zhao C Baron W . Fibronectin in tissue regeneration: timely disassembly of the scaffold is necessary to complete the build. Cell Mol Life Sci (2013) 70:4243–53. 10.1007/s00018-013-1350-0
41.
Everts P Onishi K Jayaram P Lana JF Mautner K . Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int J Mol Sci (2020) 21:7794. 10.3390/ijms21207794
42.
Cheng PG Yang KD Huang LG Wang CH Ko WS . Comparisons of cytokines, growth factors and clinical efficacy between platelet-rich plasma and autologous conditioned serum for knee osteoarthritis management. Biomolecules (2023) 13:555. 10.3390/biom13030555
43.
Zhang Z Liu P Xue X Zhang Z Wang L Jiang Y et al The role of platelet-rich plasma in biomedicine: a comprehensive overview. iScience (2025) 28:111705. 10.1016/j.isci.2024.111705
44.
Mochizuki T Ushiki T Suzuki K Sato M Ishiguro H Suwabe T et al Elevated IL-1β and comparable IL-1 receptor antagonist levels are characteristic features of L-PRP in female college athletes compared to Male professional soccer players. Int J Mol Sci (2023) 24:17487. 10.3390/ijms242417487
45.
Kulesza A Paczek L Burdzinska A . The role of COX-2 and PGE2 in the regulation of immunomodulation and other functions of mesenchymal stromal cells. Biomedicines (2023) 11:445. 10.3390/biomedicines11020445
46.
Iberg CA Hawiger D . Natural and induced tolerogenic dendritic cells. The J Immunol (2020) 204:733–44. 10.4049/jimmunol.1901121
47.
Xie M Xiong W She Z Wen Z Abdirahman AS Wan W et al Immunoregulatory effects of stem cell-derived extracellular vesicles on immune cells. Front Immunol (2020) 11–2020. 10.3389/fimmu.2020.00013
48.
Manole CG Soare C Ceafalan LC Voiculescu VM . Platelet-rich plasma in dermatology: new insights on the cellular mechanism of skin repair and regeneration. Life (Basel) (2023) 14:40. 10.3390/life14010040
49.
Pak I Askarov M Klyuyev D Tak MS Batenova U Yeskermessov D et al PRP pre-treatment of the implantation zone improves the survival rate of fat autograft. Front Bioeng Biotechnol (2025) 13–2025. 10.3389/fbioe.2025.1545419
50.
Milano G Sánchez M Jo CH Saccomanno MF Thampatty BP Wang JHC . Platelet-rich plasma in orthopaedic sports medicine: state of the art. J ISAKOS (2019) 4:188–95. 10.1136/jisakos-2019-000274
51.
Xu P Wu Y Zhou L Yang Z Zhang X Hu X et al Platelet-rich plasma accelerates skin wound healing by promoting re-epithelialization. Burns and Trauma (2020) 8:tkaa028. 10.1093/burnst/tkaa028
52.
Thu AC . The use of platelet-rich plasma in management of musculoskeletal pain: a narrative review. J Yeungnam Med Sci (2022) 39:206–15. 10.12701/jyms.2022.00290
53.
Şahin MM Cayonu M Dinc SK Ozkocer E Ilhan M Uzunoğlu E et al Effects of chitosan and platelet-rich plasma on facial nerve regeneration in an animal model. Eur Arch Otorhinolaryngol (2022) 279:987–94. 10.1007/s00405-021-06859-6
54.
Li L Cai J Yuan Y Mao Y Xu L Han Y et al Platelet-rich plasma can release nutrient factors to promote facial nerve crush injury recovery in rats. Saudi Med J (2019) 40:1209–17. 10.15537/smj.2019.12.24747
55.
Yuan B Zheng X Wu ML Yang Y Chen JW Gao HC et al Platelet-rich plasma gel-loaded collagen/chitosan composite film accelerated rat sciatic nerve injury repair. ACS Omega (2023) 8:2931–41. 10.1021/acsomega.2c05351
56.
Wang YS Wang SL Liu XL Kang ZC . Platelet-rich plasma promotes peripheral nerve regeneration after sciatic nerve injury. Neural Regen Res (2023) 18:375–81. 10.4103/1673-5374.346461
57.
Khaled MM Ibrahium AM Abdelgalil AI El-Saied MA Yassin AM Abouquerin N et al Efficacy of using adipose-derived stem cells and PRP on regeneration of 40 -mm long sciatic nerve defect bridged by polyglycolic-polypropylene mesh in canine model. Stem Cell Res Ther (2024) 15:212. 10.1186/s13287-024-03796-z
58.
Hama S Yokoi T Orita K Uemura T Takamatsu K Okada M et al Peripheral nerve regeneration by bioabsorbable nerve conduits filled with platelet-rich fibrin. Clin Neurol Neurosurg (2024) 236:108051. 10.1016/j.clineuro.2023.108051
59.
Yi D Zhang Y Li M Chen J Chen X Wang L et al Ultrasound-targeted microbubble destruction assisted delivery of platelet-rich plasma-derived exosomes promoting peripheral nerve regeneration. Tissue Eng A (2023) 29:645–62. 10.1089/ten.tea.2023.0133
60.
Zhu Y Peng N Wang J Jin Z Zhu L Wang Y et al Peripheral nerve defects repaired with autogenous vein grafts filled with platelet-rich plasma and active nerve microtissues and evaluated by novel multimodal ultrasound techniques. Biomater Res (2022) 26:24. 10.1186/s40824-022-00264-8
61.
Rahmi RD Radithia D Soebadi B Parmadiati AE Winias S . Nerve growth factor and S100B: molecular marker of neuroregeneration after injection of freeze-dried platelet rich plasma. J Oral Biol Craniofac Res (2022) 12:570–4. 10.1016/j.jobcr.2022.07.006
62.
Kim JW Kim JM Choi ME Jeon EJ Park JM Kim YM et al Platelet-rich plasma loaded nerve guidance conduit as implantable biocompatible materials for recurrent laryngeal nerve regeneration. NPJ Regen Med (2022) 7:49. 10.1038/s41536-022-00239-2
63.
Behroozi Z Ramezani F Nasirinezhad F . Human umbilical cord blood-derived platelet -rich plasma: a new window for motor function recovery and axonal regeneration after spinal cord injury. Physiol and Behav (2022) 252:113840. 10.1016/j.physbeh.2022.113840
64.
Behroozi Z Ramezani F Janzadeh A Rahimi B Nasirinezhad F . Platelet-rich plasma in umbilical cord blood reduces neuropathic pain in spinal cord injury by altering the expression of ATP receptors. Physiol and Behav (2021) 228:113186. 10.1016/j.physbeh.2020.113186
65.
Lu CF Wang B Zhang PX Han S Pi W Kou YH et al Combining chitin biological conduits with small autogenous nerves and platelet-rich plasma for the repair of sciatic nerve defects in rats. CNS Neurosci Ther (2021) 27:805–19. 10.1111/cns.13640
66.
Vares P Dehghan MM Bastami F Biazar E Shamloo N Heidari Keshel S et al Effects of platelet-rich fibrin/collagen membrane on sciatic nerve regeneration. J Craniofac Surg (2021) 32:794–8. 10.1097/scs.0000000000007003
67.
Bo Feng YZ Wu L Zhang L Shan Y . Efficacy of autologous epineurium small gap coaptation combined with platelet-rich plasma, nerve growth factor, and nerve fragments in the repair of damaged peripheral nerves. Int J Clin Exp Med (2020) 13:2902–13.
68.
Chuang MH Ho LH Kuo TF Sheu SY Liu YH Lin PC et al Regenerative potential of platelet-rich fibrin releasate combined with adipose tissue-derived stem cells in a rat sciatic nerve injury model. Cell Transpl (2020) 29:096368972091943. 10.1177/0963689720919438
69.
Salarinia R Hosseini M Mohamadi Y Ghorbani A Alamdari DH Mafinezhad A et al Combined use of platelet-rich plasma and adipose tissue-derived mesenchymal stem cells shows a synergistic effect in experimental spinal cord injury. J Chem Neuroanat (2020) 110:101870. 10.1016/j.jchemneu.2020.101870
70.
Samadian H Ehterami A Sarrafzadeh A Khastar H Nikbakht M Rezaei A et al Sophisticated polycaprolactone/gelatin nanofibrous nerve guided conduit containing platelet-rich plasma and citicoline for peripheral nerve regeneration: in vitro and in vivo study. Int J Biol Macromolecules (2020) 150:380–8. 10.1016/j.ijbiomac.2020.02.102
71.
Wang Z Mudalal M Sun Y Liu Y Wang J Wang Y et al The effects of leukocyte-platelet rich fibrin (L-PRF) on suppression of the expressions of the pro-inflammatory cytokines, and proliferation of schwann cell, and neurotrophic factors. Sci Rep (2020) 10:2421. 10.1038/s41598-020-59319-2
72.
Ikumi A Hara Y Yoshioka T Kanamori A Yamazaki M . Effect of local administration of platelet-rich plasma (PRP) on peripheral nerve regeneration: an experimental study in the rabbit model. Microsurgery (2018) 38:300–9. 10.1002/micr.30263
73.
Ikumi A Hara Y Okano E Kohyama S Arai N Taniguchi Y et al Intraoperative local administration of platelet-rich plasma (PRP) during neurolysis surgery for the treatment of digital nerve crush injury. Case Rep Orthopedics (2018) 2018:1–6. 10.1155/2018/1275713
74.
Torul D Bereket MC Onger ME Altun G . Comparison of the regenerative effects of platelet-rich fibrin and plasma rich in growth factors on injured peripheral nerve: an experimental study. J Oral Maxillofacial Surg (2018) 76:1823.e1–1823.e12. 10.1016/j.joms.2018.04.012
75.
Bayram B Akdeniz SS Diker N Helvacioğlu F Erdem SR . Effects of platelet-rich fibrin membrane on sciatic nerve regeneration. J Craniofac Surg (2018) 29:e239–e243. 10.1097/scs.0000000000004256
76.
Imam SS Al-Abbasi FA Hosawi S Afzal M Nadeem MS Ghoneim MM et al Role of platelet rich plasma mediated repair and regeneration of cell in early stage of cardiac injury. Regenerative Ther (2022) 19:144–53. 10.1016/j.reth.2022.01.006
77.
Shen YP Li TY Chou YC Chen LC Wu YT . Outcome predictors of platelet-rich plasma injection for moderate carpal tunnel syndrome. Int J Clin Pract (2021) 75:e14482. 10.1111/ijcp.14482
78.
Mourad SI Al-Dubai SA Elsayed SA El-Zehary RR . Efficacy of platelet-rich fibrin and tacrolimus on facial nerve regeneration: an animal study. Int J Oral Maxillofacial Surg (2022) 51:279–87. 10.1016/j.ijom.2021.05.016
79.
Ali S Sun M Khan MN Qiang F . Advances in sciatic nerve regeneration: a review of contemporary techniques. Regenerative Ther (2025) 29:563–74. 10.1016/j.reth.2025.04.016
80.
Yadav A Ramasamy TS Lin SC Chen SH Lu J Liu YH et al Autologous platelet-rich growth factor reduces M1 macrophages and modulates inflammatory microenvironments to promote sciatic nerve regeneration. Biomedicines (2022) 10:1991. 10.3390/biomedicines10081991
81.
Kokkalas N Kokotis P Diamantopoulou K Galanos A Lelovas P Papachristou DJ et al Platelet-rich plasma and mesenchymal stem cells local infiltration promote functional recovery and histological repair of experimentally transected sciatic nerves in rats. Cureus (2020) 12:e8262. 10.7759/cureus.8262
82.
Dong Q Yang X Liang X Liu J Wang B Zhao Y et al Composite hydrogel conduit incorporated with platelet-rich plasma improved the regenerative microenvironment for peripheral nerve repair. ACS Appl Mater Inter (2023) 15:24120–33. 10.1021/acsami.3c02548
83.
Gao N Yan L Ai F Kang J Wang L Weng Y . Comparison of the short-term clinical effectiveness of 5% dextrose water, platelet-rich plasma and corticosteroid injections for carpal tunnel syndrome: a systematic review and network meta-analysis of randomized controlled trials. Arch Phys Med Rehabil (2023) 104:799–811. 10.1016/j.apmr.2022.11.009
84.
Senna MK Shaat RM Ali AAA . Platelet-rich plasma in treatment of patients with idiopathic carpal tunnel syndrome. Clin Rheumatol (2019) 38:3643–54. 10.1007/s10067-019-04719-7
85.
Chen SR Shen YP Ho TY Li TY Su YC Chou YC et al One-year efficacy of platelet-rich plasma for moderate-to-severe carpal tunnel syndrome: a prospective, randomized, double-blind, controlled trial. Arch Phys Med Rehabil (2021) 102:951–8. 10.1016/j.apmr.2020.12.025
86.
Park J Kim J Jeon W Kim D Rhyu I Kim Y et al An inside-out vein graft filled with platelet-rich plasma for repair of a short sciatic nerve defect in rats. Neural Regen Res (2014) 9:1351–7. 10.4103/1673-5374.137587
87.
Lichtenfels M Colomé L Sebben AD Braga‐Silva J . Effect of platelet rich plasma and platelet rich fibrin on sciatic nerve regeneration in a rat model. Microsurgery (2013) 33:383–90. 10.1002/micr.22105
88.
Ye F Li H Qiao G Chen F Tao H Ji A et al Platelet-rich plasma gel in combination with schwann cells for repair of sciatic nerve injury. Neural Regen Res (2012) 7:2286–92. 10.3969/j.issn.1673-5374.2012.29.007
89.
Emel E Ergün SS Kotan D Gürsoy EB Parman Y Zengin A et al Effects of insulin-like growth factor-I and platelet-rich plasma on sciatic nerve crush injury in a rat model. J Neurosurg (2011) 114:522–8. 10.3171/2010.9.JNS091928
90.
Kuo YC Lee CC Hsieh LF . Ultrasound-guided perineural injection with platelet-rich plasma improved the neurophysiological parameters of carpal tunnel syndrome: a case report. J Clin Neurosci (2017) 44:234–6. 10.1016/j.jocn.2017.06.053
91.
Nikolaou V Malahias M Johnson E Babis G . Single injection of platelet-rich plasma as a novel treatment of carpal tunnel syndrome. Neural Regen Res (2015) 10:1856–9. 10.4103/1673-5374.165322
92.
Park GY Kwon DR . Platelet-rich plasma limits the nerve injury caused by 10% dextrose in the rabbit median nerve. Muscle Nerve (2014) 49:56–60. 10.1002/mus.23863
93.
Güven SC Özçakar L Kaymak B Kara M Akıncı A . Short-term effectiveness of platelet-rich plasma in carpal tunnel syndrome: a controlled study. J Tissue Eng Regen Med (2019) 13:709–14. 10.1002/term.2815
94.
Anitua E Troya M Falcon-Pérez JM López-Sarrio S González E Alkhraisat MH . Advances in platelet rich plasma-derived extracellular vesicles for regenerative medicine: a systematic-narrative review. Int J Mol Sci (2023) 24:13043. 10.3390/ijms241713043
95.
Berrigan WA Bailowitz Z Park A Reddy A Liu R Lansdown D . A greater platelet dose May yield better clinical outcomes for platelet-rich plasma in the treatment of knee osteoarthritis: a systematic review. Arthrosc The J Arthroscopic and Relat Surg (2025) 41:809–17. e2. 10.1016/j.arthro.2024.03.018
96.
Boffa A De Marziani L Andriolo L Di Martino A Romandini I Zaffagnini S et al Influence of platelet concentration on the clinical outcome of platelet-rich plasma injections in knee osteoarthritis. The Am J Sports Med (2024) 52:3223–31. 10.1177/03635465241283463
97.
Zhang K Zhang C Ren Q Wang D Sun L Wang X et al Effects of Leukocyte-rich platelet-rich plasma and Leukocyte-poor platelet-rich plasma on cartilage in a rabbit osteoarthritis model. Cell Mol Biol (2024) 70:217–26. 10.14715/cmb/2024.70.2.31
98.
Di Martino A Boffa A Andriolo L Romandini I Altamura SA Cenacchi A et al Leukocyte-rich versus leukocyte-poor platelet-rich plasma for the treatment of knee osteoarthritis: a double-blind randomized trial. The Am J Sports Med (2022) 50:609–17. 10.1177/03635465211064303
99.
Yuan Z Wang Y Li Y Lin C Wang S Wang J et al Comparison of leukocyte-rich and leukocyte-poor platelet-rich plasma on pressure ulcer in a rat model. J Burn Care and Res (2023) 44:860–8. 10.1093/jbcr/irac191
100.
Lana JF Huber SC Purita J Tambeli CH Santos GS Paulus C et al Leukocyte-rich PRP versus leukocyte-poor PRP-the role of monocyte/macrophage function in the healing Cascade. J Clin Orthopaedics Trauma (2019) 10:S7–S12. 10.1016/j.jcot.2019.05.008
101.
Simental-Mendía M Ortega-Mata D Tamez-Mata Y Olivo CAA Vilchez-Cavazos F . Comparison of the clinical effectiveness of activated and non-activated platelet-rich plasma in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Clin Rheumatol (2023) 42:1397–408. 10.1007/s10067-022-06463-x
102.
Smith OJ Talaat S Tomouk T Jell G Mosahebi A . An evaluation of the effect of activation methods on the release of growth factors from platelet-rich plasma. Plast and Reconstr Surg (2022) 149:404–11. 10.1097/prs.0000000000008772
103.
Gentile P Calabrese C De Angelis B Dionisi L Pizzicannella J Kothari A et al Impact of the different preparation methods to obtain autologous non-activated platelet-rich plasma (A-PRP) and activated platelet-rich plasma (AA-PRP) in plastic surgery: wound healing and hair regrowth evaluation. Int J Mol Sci (2020) 21:431. 10.3390/ijms21020431
104.
Cavallo C Roffi A Grigolo B Mariani E Pratelli L Merli G et al Platelet‐rich plasma: the choice of activation method affects the release of bioactive molecules. Biomed Research International (2016) 2016:1–7. 10.1155/2016/6591717
105.
Li M Zhao Y Chen X Du X Luo Y Li Y et al Comparative analysis of the quality of platelet concentrates produced by apheresis procedures, platelet rich plasma, and buffy coat. Transfusion (2024) 64:367–79. 10.1111/trf.17704
106.
Carvalho A Ferreira AF Soares M Santos S Tomé P Machado-Simões J et al Optimization of platelet-rich plasma preparation for regenerative medicine: comparison of different anticoagulants and resuspension media. Bioengineering (2024) 11:209. 10.3390/bioengineering11030209
107.
Zhang EJX Lie VE Wong KLF Zhang E . Impact of centrifugation parameters on platelet-rich plasma injection for patella tendinopathy: a systematic review and meta-analysis. Cureus (2024) 16:e63341. 10.7759/cureus.63341
108.
Castillo-Macías A Zavala J Ortega-Lara W García-Herrera S Valdez-García J . Optimizing platelet and leucocyte-rich plasma as biomaterials for ophthalmic applications: impact of centrifugation speed. Clin Ophthalmol (2023) Vol. 17:3787–97. 10.2147/opth.s444840
109.
Harrison TE Bowler J Cheng C-I Reeves KD . Optimizing platelet-rich plasma: spin time and sample source. Bioengineering (2023) 10:1270. 10.3390/bioengineering10111270
110.
Gill TJ . CORR insights®: there is wide variation in platelet-rich plasma injection pricing: a United States nationwide study of top orthopaedic hospitals. Clin Orthop Relat Res (2024) 482:685–7. 10.1097/corr.0000000000002940
111.
Garcia RP Brodt FP Marchi CMD Barja PR Doria ACOC Furtado GRD et al Comparative study on the efficacy of injectable platelet rich fibrin (i-PRF) and albumin gel (ALB-Gel) in facial rejuvenation: a clinical ultrasonographic evaluation. J Adv Med Med Res (2024) 36:50–64. 10.9734/jammr/2024/v36i45398
112.
Chen L Jin S Yao Y He S He J . Comparison of clinical efficiency between intra-articular injection of platelet-rich plasma and hyaluronic acid for osteoarthritis: a meta-analysis of randomized controlled trials. Ther Adv Musculoskelet Dis (2023) 15:1759720X231157043. 10.1177/1759720x231157043
113.
Xiong Y Gong C Peng X Liu X Su X Tao X et al Efficacy and safety of platelet-rich plasma injections for the treatment of osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Front Med (2023) 10:1204144. 10.3389/fmed.2023.1204144
114.
Zhou Y Li H Cao S Han Y Shao J Fu Q et al Clinical efficacy of intra‐articular injection with P‐PRP versus that of L‐PRP in treating knee cartilage lesion: a randomized controlled trial. Orthopaedic Surg (2023) 15:740–9. 10.1111/os.13643
115.
Mariani E Pulsatelli L Cattini L Dolzani P Assirelli E Cenacchi A et al Pure platelet and Leukocyte–platelet-rich plasma for regenerative medicine in Orthopedics—Time-and preparation-dependent release of growth factors and effects on synovial fibroblasts: a comparative analysis. Int J Mol Sci (2023) 24:1512. 10.3390/ijms24021512
116.
Barbieri M Colombini A Stogicza A de Girolamo L . Effectiveness of plasma rich in growth factors in the management of chronic spinal pain: a case series of 32 patients. Regenerative Med (2022) 17:175–84. 10.2217/rme-2021-0128
117.
Park Y-B Kim J-H Ha C-W Lee D-H . Clinical efficacy of platelet-rich plasma injection and its association with growth factors in the treatment of mild to moderate knee osteoarthritis: a randomized double-blind controlled clinical trial as compared with hyaluronic acid. The Am J Sports Med (2021) 49:487–96. 10.1177/0363546520986867
118.
Sánchez M Jorquera C López de Dicastillo L Martínez N Espregueira‐Mendes J Vergés J et al Women show a positive response to platelet‐rich plasma despite presenting more painful knee osteoarthritis than men. Knee Surg Sports Traumatol Arthrosc (2024) 32:2516–25. 10.1002/ksa.12080
119.
Argut SK Celik D Ergin ON Kilicoglu OI . Factors affecting the features of platelet-rich plasma in patients with knee osteoarthritis. Acta Orthopaedica et Traumatologica Turcica (2023) 57:148–53. 10.5152/j.aott.2023.22077
120.
Rossi L Ranalletta M Pasqualini I Zicaro JP Paz MC Camino P et al Substantial variability in platelet-rich plasma composition is based on patient age and baseline platelet count. Arthrosc Sports Med Rehabil (2023) 5:e853–e858. 10.1016/j.asmr.2023.03.017
121.
Zahir H Dehghani B Yuan X Chinenov Y Kim C Burge A et al In vitro responses to platelet-rich-plasma are associated with variable clinical outcomes in patients with knee osteoarthritis. Scientific Rep (2021) 11:11493. 10.1038/s41598-021-90174-x
122.
Egierska D Perszke M Mazur M Duś-Ilnicka I . Platelet-rich plasma and platelet-rich fibrin in oral surgery: a narrative review. Dental Med Probl (2023) 60:177–86. 10.17219/dmp/147298
123.
Gupta A Maffulli N Jain VK . Red blood cells in platelet-rich plasma: avoid if at all possible. Biomedicines (2023) 11:2425. 10.3390/biomedicines11092425
124.
Chowdhary K Sahu A Iijima H Shinde S Borg-Stein J Ambrosio F . Aging affects the efficacy of platelet-rich plasma treatment for osteoarthritis. Am J Phys Med Rehabil (2023) 102:597–604. 10.1097/phm.0000000000002161
125.
Taniguchi Y Yoshioka T Sugaya H Gosho M Aoto K Kanamori A et al Growth factor levels in leukocyte-poor platelet-rich plasma and correlations with donor age, gender, and platelets in the Japanese population. J Exp Orthopaedics (2019) 6:4. 10.1186/s40634-019-0175-7
126.
Tian J Li XJ Ma Y Mai Z Yang Y Luo M et al Correlation of bioactive components of platelet rich plasma derived from human female adult peripheral blood and umbilical cord blood with age. Sci Rep (2023) 13:18428. 10.1038/s41598-023-45747-3
127.
Verma R Kandwal A Negi G Chandra H . Factors affecting the quantity and quality of platelet-rich plasma and platelet-derived growth factor-BB: an observational study. J Bio-X Res (2021) 04:67–70. 10.1097/jbr.0000000000000091
128.
Rizki RR Benni BR Riki RM . Growth factor identification based on speed and duration of centrifugation in platelet rich plasma. Front Healthc Res (2024) 1:69–75. 10.63918/fhr.v1.n1.p69-75.2024
129.
Goodale MB Phelps HA Barnhard JA Shoben AB Brunke MW . Lower centrifugation speed and time are positively associated with platelet concentration in a canine autologous conditioned plasma system. J Am Vet Med Assoc (2023) 261:1–6. 10.2460/javma.23.04.0218
130.
Li H Xia T Zeng H Qiu Y Wei Y Cheng Y et al Liquid platelet-rich fibrin produced via horizontal centrifugation decreases the inflammatory response and promotes chondrocyte regeneration in vitro. Front Bioeng Biotechnol (2023) 11:1301430. 10.3389/fbioe.2023.1301430
131.
Legiawati L Yusharyahya SN Bernadette I Novianto E Priyanto MH Gliselda KC et al Comparing single-spin versus double-spin platelet-rich plasma (PRP) centrifugation methods on thrombocyte count and clinical improvement of androgenetic alopecia: a preliminary, randomized, double-blind clinical trial. J Clin Aesthet Dermatol (2023) 16:39–44.
132.
Muthu S Krishnan A Ramanathan KR . Standardization and validation of a conventional high yield platelet-rich plasma preparation protocol. Ann Med Surg (2012) (2022) 82:104593. 10.1016/j.amsu.2022.104593
133.
Pretorius J Habash M Ghobrial B Alnajjar R Ellanti P . Current status and advancements in platelet-rich plasma therapy. Cureus (2023) 15:e47176. 10.7759/cureus.47176
134.
Arita A Tobita M . Adverse events related to platelet-rich plasma therapy and future issues to be resolved. Regenerative Ther (2024) 26:496–501. 10.1016/j.reth.2024.07.004
Summary
Keywords
peripheral nerve injury, platelet-rich plasma, nerve regeneration, growth factors, neurotrophic factors, schwann cells, therapeutic target, regenerative medicine
Citation
Shang K, Liu Y and Qadeer A (2025) Platelet-rich plasma in peripheral nerve injury repair: a comprehensive review of mechanisms, clinical applications, and therapeutic potential. Exp. Biol. Med. 250:10746. doi: 10.3389/ebm.2025.10746
Received
11 July 2025
Accepted
08 September 2025
Published
23 September 2025
Volume
250 - 2025
Updates
Copyright
© 2025 Shang, Liu and Qadeer.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kai Shang, kkai806@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.