Abstract
Berberine, known as an antioxidant agent, can improve glycemic indices in animal models of diabetes; however, it is clinically limited by poor bioavailability. Nanoparticles show the desirable capacity as delivery platforms for improving the bioavailability of medicinal agents. Here, we aimed to enhance the bioavailability and therapeutic impacts of berberine in streptozotocin (STZ)-induced gestational diabetes mellitus (GDM) rats by its encapsulation into the chitosan-coated solid lipid nanoparticles (SLNs) formulation. Berberine-loaded chitosan/SLN nanoparticles were formulated by the solvent-injection approach followed by a homogenization operation. The particle size, surface charge, and polydispersity index, as well as encapsulation efficiency percent (EE%), in vitro stability and berberine release, and in vivo pharmacokinetics were studied. Glycemic indices, such as fasting glucose and insulin, oral glucose tolerance, insulin tolerance, and homeostasis model of insulin resistance (HOMA-IR) scores, as well as the activity level of liver antioxidant and pro-oxidant enzymes, were evaluated in STZ-induced GDM rats. The particle size of berberine-loaded chitosan/SLN formulation was detected in the nano-range with high stability and high EE% as well as a sustained-release profile. Berberine nanoparticle treatment could provide a significantly higher oral bioavailability of berberine in experimental rats. Berberine nanoparticles remarkably reversed the altered glycemic indices, body weight, and pro-oxidant/antioxidant balance in STZ-induced GDM rats, with significantly higher effects than free berberine. In conclusion, chitosan-coated SLN nanoparticles firmly enhanced the therapeutic impacts of berberine on STZ-induced GDM, suggesting chitosan-coated SLN nanoparticles as an efficient oral delivery system for enhancing the bioavailability of berberine and, thus, improving its pharmacological impacts.
Graphical Abstract
Impact statement
Chitosan-coated SLN nanoparticles provide an efficient oral delivery system to enhance oral bioavailability of berberine and, thus, improve its pharmacological impacts.
Introduction
Diabetes Mellitus is a chronic metabolic disease manifested via increased concentration of blood glucose through impaired glucose, protein, and lipid metabolism due to insulin deficiency, which can result from impaired production of insulin by the pancreas gland’s Langerhans beta cells and/or result from insulin resistance by unresponsiveness of the body’s cells to insulin. It is a leading cause of morbidity and mortality, particularly in pregnant women, termed gestational diabetes mellitus (GDM). GDM shows various degrees of glucose intolerance in pregnant women, leading to chronic hyperglycemia that causes long-term injuries, including the development of pre-eclampsia, gestational hypertension, liver damage, and kidney damage. The pathological feature of GDM, like type 2 diabetes mellitus (T2DM), is correlated with both defective insulin secretion and insulin resistance. Notably, normal pregnancy requires elevated glucose production and decreased insulin sensitivity to provide the energy required for the fetus; thus, there is an association between pregnancy and the progression of maternal insulin resistance [1]. During pregnancy, insulin resistance occurs because of the secretion of placental hormones that antagonize insulin. Therefore, during pregnancy, the body needs a high demand for insulin, which increases the load on the pancreatic β-cells, leading to β-cells exhaustion and consequently reduced insulin production [2]. Of note, oxidative stress, an imbalance between the body’s antioxidant defense mechanisms and the generation of free radicals, is an important contributor to the development and pathogenesis of GDM during pregnancy. The increased level of glucose during the GDM condition leads to increased formation of highly toxic oxygen and hydroxyl free radicals through glucose metabolism, such as glucose auto-oxidation, metabolism of methylglyoxal formation, and oxidative phosphorylation [3]. Using antidiabetic drugs (mainly metformin) orally or insulin injection is the main treatment in GDM. However, such treatments are expensive, and long-term use of antidiabetic medications can cause serious adverse impacts on the various organs. In addition, although the insulin therapy can control the blood glucose, it has no therapeutic impact on the function of β-cells and the insulin resistance [4]. On the other hand, targeting oxidative stress may inhibit the GDM-related pathogenesis, suggesting antioxidant agents as potential protective treatment against diabetes complications by reducing free radicals.
Berberine, as a plant-derived medicinal compound, is the major active ingredient detected in the stem, bark, roots, and rhizome of many plants, including barberry (Berberis vulgaris), tree turmeric (Berberis aristata), Oregon grape (Berberis aquifolium), Coptis (Coptis chinensis), and goldenseal (Hydrastis canadensis) [5]. Berberine is mainly known for its antioxidant property, through which it exerts protective impacts on various diseases in preclinical and clinical settings, such as diabetes, cancer, infection, and cardiovascular diseases [6–15]. An increasing body of research has recently shown that berberine can exert effective hypoglycemic activity and, thereby, provide an improving effect on diabetic complications, including diabetic cardiomyopathy, diabetic neuropathy, diabetic nephropathy, diabetic encephalopathy, as well as GDM [11, 16–22]. Interestingly, berberine is found to have a hypoglycemic impact similar to rosiglitazone and metformin in patients with T2DM [23, 24]. Several meta-analyses of clinical trials in T2DM patients revealed that berberine has good safety and a low incidence of total adverse events, and berberine administered alone or in combination with oral hypoglycemic drugs is effective in the treatment of T2DM patients and firmly regulates postprandial blood glucose, fasting blood glucose, and HbA1c [11, 18–20]. Several preclinical investigations have recently shown potential of berberine in the GDM treatment, where it was found to improve glucose tolerance, insulin response, body and fetal weight, placental weight, as well as the number of dead and absorptive fetuses in the experimental models of GDM [21, 25–28]. Berberine has been found to exert the glucose-lowering impact by multiple mechanisms, such as elevating glucose uptake, inhibiting gluconeogenesis, enhancing glycolysis, improving insulin secretion and insulin response, and suppressing the action of important enzymes involved in the carbohydrate digestion in the digestive tract (such as α-glucosidase and α-amylase), and improving the anti-oxidant and anti-inflammatory responses [18, 29, 30]. Although berberine has shown a wide range of therapeutic benefits, its oral use is clinically limited because of poor aqueous solubility and intestinal absorption, rapid metabolism by the first-pass effect in the liver and intestine, as well as short biological half-life, which cause low blood concentrations and poor bioavailability. In addition, berberine at high doses causes gastrointestinal side effects, such as cramping and stomach upset, because of its low intestinal absorption and long-term administration [31–33]. Therefore, manufacturing a delivery system to provide a sustained release of berberine is required to improve its bioavailability by reducing the dissolution rate in the gastric environment and increasing the residence time in the intestinal mucus.
Solid lipid nanoparticles (SLNs) have been used as a delivery system to enhance the bioavailability and therapeutic efficiency of natural compounds [34]. In recent years, SLNs have received a significant considerable attention because of their interesting advantages, such as low toxicity and immunogenicity, increasing solubility and bioavailability of both hydrophobic and hydrophilic drugs, triggering pharmacological property of drugs, protecting the drugs against digestive enzymes, having excellent biodegradability and biocompatibility, as well as providing an easy and low-price formulation [35]. The important criteria impacting the in vivo efficacy of the oral drug delivery formulations are their integrity and stability in gastrointestinal media. Despite a high stability against digestive enzymes, the low stability of SLNs in the stomach’s acidic pH limits their clinical utilization. For resolving this issue, coating of SLNs by the biopolymers, such as chitosan, with a significant resistance to the acidic media and a high mucoadhesive property, has been employed [36]. Chitosan is a chitin-derived cationic polysaccharide that, under acidic pH, is protonated and strongly binds to the negatively charged SLNs, thereby providing a stable delivery system with the ability to sustainably release an encapsulated drug [37]. Further, when chitosan is coated on the nanoparticle surface, it can improve the absorption of encapsulated drugs via a mucosal surface [38]. Therefore, formulating SLNs with chitosan can protect them against the acidic gastrointestinal medium and enhance their transmucosal delivery, consequently increasing the effective concentration of encapsulated drugs at the absorption site. Of note, chitosan-coated SLN nanoparticles have been found to remarkably improve the oral bioavailability of numerous encapsulated drugs in vivo by elevating residence time at mucosa due to their enhanced mucoadhesive property [37, 39–41]. Here, we aimed to bring together biological properties of SLNs and chitosan to manufacture a nanoparticle-based carrier system for oral delivery of berberine, and to determine ameliorating impact of the prepared berberine-loaded nanoparticles on glycemic indices and prooxidant/antioxidant balance in streptozotocin (STZ)-induced GDM rats.
Materials and methods
Manufacturing berberine-loaded chitosan/SLN nanoparticles
Berberine-loaded chitosan/SLN nanoformulation was prepared using a previously described solvent-injection method [42] followed by a high-pressure homogenization process [43]. Briefly, berberine (50 mg) and the solid lipid Witepsol 85E (40 mg) were dissolved at 75 °C in 1 mL of a mixed solution (acetone and ethanol in an equal ratio (1:1 v/v)) until a homogenous dispersion appeared. To construct berberine-loaded chitosan-coated SLN nanoparticles, prepared solid lipid/berberine mix was injected into hot double-distilled water (75 °C) containing chitosan (0.5 mg/mL) and a stabilizing surfactant (Pluronic, 2.5 mg/mL). The prepared solution was emulsified by high agitation (25,000 rpm for 4 min at 70 °C) and then homogenized at the same temperature through employing nine homogenization cycles at 750 bars by a high-pressure homogenizer. Immediately after homogenization, the obtained suspension was cooled in an ice-water bath to keep the structure of berberine-loaded chitosan/SLN nanoparticles stable. Finally, the prepared nanoformulation was filtered by a 400 nm filter and then freeze-dried.
Characterization of the manufactured berberine nano-formulation
Physiochemical analysis
Physicochemical indexes of the constructed nanoformulation were studied by measuring the particle size (Z-average diameter), zeta potential (surface charge), and polydispersity index (PDI) by a dynamic light scattering instrument (Zetasizer, UK) at 25 °C.
Encapsulation efficiency
To measure the amount of berberine entrapment in the chitosan-coated SLN nano-formulation, the encapsulation efficiency (EE) percentage was evaluated by determining the unloaded (free) berberine concentration through the centrifugal ultrafiltration method, followed by the high-performance liquid chromatography (HPLC) technique. To isolate free berberine, the berberine-loaded SLN/chitosan nano-formulation was centrifuged for 30 min at 23,000 rpm and 4 °C. Afterward, the free berberine amount in the liquid supernatant was determined via HPLC on a Shimadzu system (CBM 20A) equipped with a C18 reverse-phase column (4.6 mm × 25 cm) and a multi-channel UV–VIS detector. To determine the concentration of berberine in the test samples, the HPLC standard curve was made based on the standard solutions containing known concentrations of berberine. Eventually, the nanoparticle EE (%) was determined from the amount of entrapped berberine to the primary loaded berberine. The entrapped concentration of berberine was measured by subtracting berberine amount detected by HPLC from the initially added amount of berberine. The following equation was used to calculate EE (%) [44]: EE (%) = (C0 - C)/C0 × 100%, where C0 is the amount of berberine primary loaded into nano-formulation, and C is the amount of free berberine.
In vitro stability
The stability of berberine-loaded chitosan/SLN nanoparticles was determined within the simulated gastric fluid (SGF, pH 1.5) and simulated intestinal fluid (SIF, pH 6.8). Nanoparticles were loaded into the SGF and SIF, and then incubated at 37 °C for 2 and 6 h, respectively. Such time intervals were used in accordance with the predicted homing times in the intestine and stomach. Particle size and EE% were evaluated during these time periods.
The SGF medium was prepared by dissolving 2 g/L of sodium chloride NaCl and 3.2 g/L pepsin in deionized water and then adjusting the pH to 1.5 ± 0.2 with 1 M hydrochloric acid (HCl). The SIF medium was prepared by dissolving 6.8 g/L of potassium phosphate monobasic (KH2PO4), 10 g/L of sodium dodecyl sulfate (SDS), and 8400 U/L of 8400 U/L in deionized water and then pH adjusted to 6.8 ± 0.2 with 1 N sodium hydroxide (NaOH) solution [45].
In vitro drug release
In vitro drug release was performed in the SGF and SIF media to simulate the physiological status through oral use. Berberine-loaded chitosan/SLN nanoparticles were incubated at 37 °C through constant shaking (100 rpm) with the SGF or SIF in micro-centrifuge tubes, individually. Nanoparticles were separated through ultrafiltration-centrifugation by Millipore tubes (MWCO = 5 kDa). Eventually, the filtered nanoparticle formulation was analyzed to determine the concentration of berberine by the previously explained HPLC method [46]. The cumulative release calculation method involved determining the percentage of berberine released over time from the nanoformulation, using the following formula [47]:
Where Pt = percentage release at time “t” and P (t – 1) = percentage release previous to “t.”
In vivo pharmacokinetic study
Berberine-loaded chitosan/SLN nanoparticles and free berberine were gavaged (50 mg/kg) in two groups (n = 10 rats per group) of female Wistar-Albino rats. Tail vein blood samples were collected and moved into K3EDTA tubes at the following times: 0.5, 1, 2, 4, 8, 12, 18, and 24-h after dosing. The plasma was then separated from the blood samples by centrifuging at 3000 rpm for 8 min at 4 °C, and stored in 1.5 mL tubes at −20 °C. Pharmacokinetic parameters, including the area under the berberine plasma versus time curve (AUC), the maximum plasma concentration of berberine after oral administration (Cmax), and the time to maximum plasma concentration of berberine (Tmax), were measured via HPLC analysis of plasma samples [48].
Gestational rats
Forty-nine female (180 ± 10 g) and seven male (230 ± 10 g) Wistar-Albino rats (8–10 weeks old) were obtained from the laboratory animal research center of the College of Science, King Saud University, Saudi Arabia. All animal experiments were performed in full accordance with the Animal Welfare instructions approved by the Institutional Ethics Committee and Research Advisory Committee of the King Saud University, Saudi Arabia (No.: A2025000127). All animals were housed in a specific pathogen-free environment in positive pressure rooms at a constant temperature of 22 ± 2 °C and relative humidity of 50–70% with a standard 12/12-h day-night cycle and fed a standard rodent diet and water ad libitum. Every possible attempt or action was taken to achieve the lowest suffering. The animals were kept in the laboratory for 1 week to acclimate to the conditions. After acclimatization, vaginal smears were carried out every day to determine the rats’ oestrous cycle, and female rats in the oestrous stage were allowed to mate with male rats by housing them at a 1:1 ratio in individual cages. The next morning, pregnancy in female rats was confirmed by the appearance of sperm in the vagina or by a copulatory plug observed using a microscope, and this day was marked as gestational day (GD) 0 [49].
Induction of gestational diabetes mellitus
After pregnancy confirmation (GD0), a single dose (50 mg/kg) of streptozotocin (STZ; Sigma–Aldrich) was intraperitoneally injected in the overnight fasted pregnant rats to induce GDM [50]. A group of pregnant rats were intraperitoneally injected with citrate buffer lacking STZ, termed the non-diabetic group. On the fourth day after STZ adminstration, fasting blood glucose (FBG) levels were quantified through the tail incision method with the glucometer (Roche Diagnostic), and pregnant rats suffering blood glucose concentrations more than 180 mg/dL were considered as GDM rats and subjected to further studies. Blood was collected in serum-free tubes through retro-orbital sinus bleeding.
Experimental design
The experimental study was carried out in seven groups, consisting of seven pregnant rats in each group, allocated by a blinded randomization. Normal Pregnant Control (NPC) group: non-diabetic pregnant rats orally gavaged with SLN/chitosan nanoparticles. Gestational Diabetes Mellitus (GDM) group: GDM rats orally gavaged with SLN/chitosan nanoparticles. LBN group: GDM rats orally gavaged with a Low dose of Berberine Nanoparticles (25 mg/kg/day, LBN). HBN group: GDM rats orally gavaged with a High dose of Berberine Nanoparticles (50 mg/kg/day, HBN). LBF group; GDM rats orally gavaged with a Low dose of Free-Berberine (25 mg/kg/day, LBF). HBF group: GDM rats orally gavaged with a High dose of Free-Berberine (50 mg/kg/day, HBF). Standard (STD) group: GDM rats orally gavaged by metformin (200 mg/kg/day). Berberine-loaded SLN/chitosan nanoformulation was administered to rats by oral gavage once per day from GD4 to GD18. Treatment doses and sample size were used in accordance with already published studies [51–55]. At the final day of the study, the rats were euthanized via intraperitoneal (i.p.) administration of thiopental sodium (30 mg/kg) [56, 57], and the liver tissue was isolated and stored at low temperature (−80 °C) for further experiments.
Body weight, fasting glucose and insulin, and HOMA-IR
Body weight, as well as the blood glucose level and the serum insulin concentration, were assayed on GD0, GD4, and GD18 after overnight fasting. The body weight of rats was measured by a top loader balance (Thermo Fisher Scientific, Inc.). The FBG level was quantified through the tail incision method with the glucometer (Roche Diagnostics). To measure the fasting insulin levels, the blood samples were obtained from the orbital venous plexus, and plasma samples were isolated by centrifugation at 12,000 rpm for 10 min at 4 °C. The level of fasting insulin was quantified in plasma samples by a rat insulin enzyme-linked immunosorbent assay (ELISA) kit (Termo Scientific). To estimate insulin resistance, the Homeostasis Model of Insulin Resistance (HOMA-IR) was measured by the following formula: HOMA-IR = (Fasting glucose × Fasting insulin)/405. The two main pathophysiological mechanisms of HOMA-IR are insulin resistance and pancreatic cell dysfunction due to insulin resistance [58].
Oral glucose tolerance test
The oral glucose tolerance test (OGTT) was employed to evaluate glucose tolerance in overnight fasted rats orally given glucose (2 g/kg) 2 weeks after treatment (G18). In brief, glucose solution was orally administered, and the blood level of glucose was detected before and 30, 60, 90, and 120 min after glucose gavage [59]. Data were analyzed as the integrated area under the curve for glucose (AUCglucose) and calculated by the trapezoid rule by GraphPad Prism version 9.
Insulin tolerance test
The insulin tolerance test (ITT) was carried out to evaluate insulin response, reflecting a value of peripheral usage of the blood glucose. After 2 weeks of treatment (G19), insulin (0.8 U/kg, i.p.) was injected to overnight fasted rats. The blood level of glucose was assessed before insulin administration (0 min) and at 30, 60, 90, and 120 min following insulin administration [60]. Results were expressed as AUCglucose.
Hepatic oxidative stress
At the end of the study, the liver tissue was isolated and homogenized at 4 °C in the saline solution (50 mM potassium phosphate, pH 7.0, containing 1 mM EDTA) and centrifuged at 10,000 g for 15 min at 4 °C. Afterward, the upper layer of the centrifuged solution was collected for assaying the hepatic status of oxidative stress parameters including super oxide dismutase (SOD; as an antioxidant catalyzing degradation of superoxide radicals into hydrogen peroxide (H2O2) and O2, catalase (CAT; as an antioxidant catalyzing degradation of H2O2 into H2O and O2), glutathione (GSH; as an antioxidant scavenging ROS), glutathione peroxidase (GPx; as an antioxidant using GSH as a cofactor to convert hydrogen peroxide and organic hydroperoxides to water and alcohol), malonaldehyde (MDA; as a biomarker of oxidative stress indicating lipid peroxidation), myeloperoxidase (MPO; as an oxidative stress biomarker reflecting liver injury), and nitric oxide (NO; as a biomarker of oxidative stress indicating liver injury), by commercial kits (Cayman Chemical Company, USA) according to the manufacturer’s protocols.
Statistical analysis
The normality testing (Shapiro-Wilk and Kolmogorov-Smirnov) showed the normal distribution of data and, thus, data were expressed as the mean ± standard deviation (SD). The between-groups significant differences were determined by the unpaired t-test or one-way ANOVA and Tukey-Kramer post-hoc multiple comparison tests (GraphPad Prism software, version 9, San Diego, CA). Data with p < 0.05 were statistically considered significant. Data were analyzed by a blinded approach.
Results
Physicochemical properties of berberine nanoparticles
The particle size analysis of Berberine-loaded chitosan/SLN nanoparticles showed a considerably narrow particle size and a notably narrow distribution width, verifying a significant dispersion quality. The prepared nanoparticles exhibited average size, surface charge, and PDI of 295 ± 12 nm, +38 ± 1.1 mV, and 0.19 ± 0.09, respectively. Of note, the PDI values < 0.3 confirm a narrow distribution of mean diameter [61]. The surface charge values allow for the prediction of the storage stability of nanoparticles; there are repulsive forces between charged particles, which prevent particle contact and agglomeration. Consequently, charged particles exhibit lower aggregation than neutral particles. The positive surface charge of Berberine-loaded SLN/chitosan nanoparticles is due to the presence of the protonated amino groups in chitosan polymer, which is suitable for obtaining a stable formulation. Additionally, the positive surface charge also confirmed surface coating of the SLN layer by the chitosan polymer. Further, EE% of berberine by chitosan-coated SLN nanoparticles was found to be 88.2 ± 2.4%, and a final lipid-to-drug ratio of 1:0.9.
In vitro stability of berberine nanoparticles
The stability of berberine-loaded chitosan/SLN nanoparticles was evaluated in SGF and SIF media, in vitro. As stated in Table 1, the prepared nanoparticles showed a notable stability in SGF and SIF. It can be attributed to the protective effect of multilayer coatings by SLN and chitosan; the SLN component protects nanoparticles against gastrointestinal digestive enzymes, and chitosan can protect the particle stability and integrity against acidic pH. Therefore, SLN and chitosan layers can protect nanoparticles from a harsh gastrointestinal environment, ensuring the robustness and stability of the prepared nanoformulation.
TABLE 1
| Medium | Particle size (nm) | Encapsulation efficiency (%) | ||||
|---|---|---|---|---|---|---|
| Pre-incubation | Post-incubation | p-value | Pre-incubation | Post-incubation | p-value | |
| SGF (pH 1.5) | 295 ± 12 | 267.4 ± 15 | 0.06 | 88.2 ± 2.4 | 63.9 ± 8 | 0.054 |
| SIF (pH 6.8) | 295 ± 12 | 279.5 ± 19 | 0.2 | 88.2 ± 2.4 | 72.7 ± 6 | 0.07 |
In vitro stability of berberine-loaded chitosan/SLN nanoparticles in SGF and SIF media.
In vitro drug release of berberine nanoparticles
The in vitro release experiment was carried out in SIF (pH 6.8) and SGF (pH 1.5) media simulating in vivo physiological status. Figure 1A shows the profile of berberine release from chitosan-coated SLN nanoparticles in SIF and SGF media. Notably, berberine loaded in chitosan/SLN nanoparticles demonstrated a much slower cumulative release than free-berberine, suggesting the effective protective effect of nanoparticles on berberine against the gastrointestinal harsh acidic environment. The release rates of berberine from chitosan/SLNs nanoparticles were found to be 82.9% ± 3.3 and 46.1% ± 4.3 after 48 h in SIF and SGF media, respectively. The release profile of berberine showed a biphasic pattern during 48 h, including an initiating phase by an early burst release pattern and a late phase by a sustained release pattern. The presence of chitosan is suitable for much slower release rates [62]. Slow releases of Berberine-loaded chitosan/SLN nanoparticles in the SIF and SGF fluids suggest that an adequately high concentration of berberine can be absorbed via the small intestine. Therefore, chitosan/SLN nanoparticles are able to endow a long-acting and effective carrier system for enhancing the intestinal absorption of orally administered berberine.
FIGURE 1
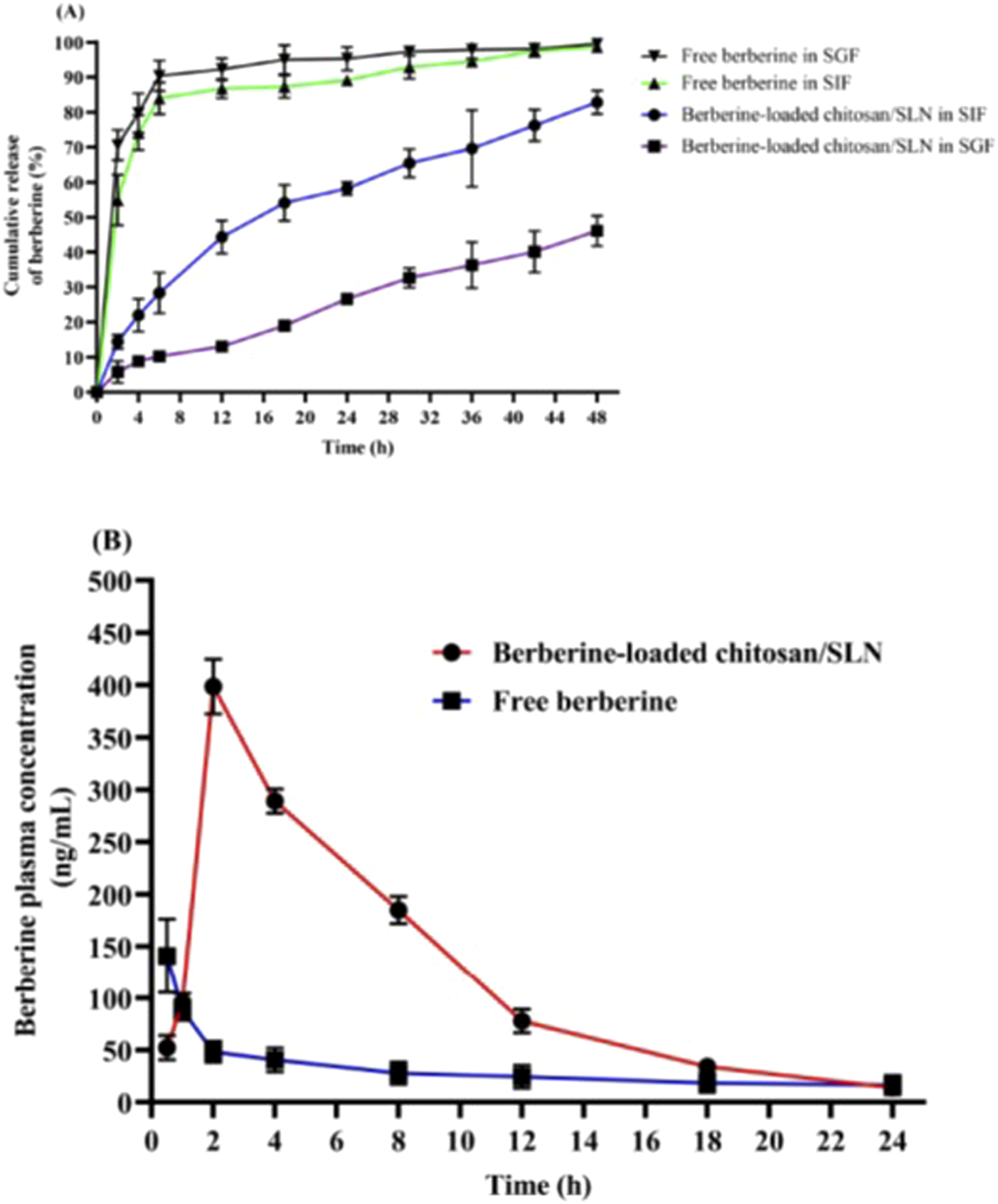
(A)In vitro release profiles of free berberine and Berberine-loaded chitosan/SLN nanoparticles in simulated gastric fluid (SGF, pH 1.5) and simulated intestinal fluid (SIF, pH 6.8) media. The data are represented as the mean ± SD (n = 3). (B) The plasma profile of berberine versus time after oral gavage of free berberine and Berberine-loaded chitosan/SLN nanoparticles in rats. The data are represented as the mean ± SD (n = 10).
In vivo pharmacokinetic study
Figure 1B shows increased blood levels of berberine 24 h after oral gavage of berberine-loaded chitosan/SLN nanoparticles. Pharmacokinetic values of Berberine-loaded chitosan/SLN nanoparticles detected in the treated rats are summarized in Table 2. The Tmax and Cmax of Berberine-loaded chitosan/SLN nanoparticles were found to be 2 h and 399 ± 26 ng/mL, respectively, whereas significantly lower Tmax (0.5 h) and Cmax (141 ± 35 ng/mL) were detected with free berberine. A higher AUC was achieved with Berberine-loaded chitosan/SLN nanoparticles (2,926 ± 75 ng/h/mL) as compared with free berberine (692 ± 74 ng/h/mL). Of note, relative bioavailability exhibited a 2.8-fold increase in the berberine nanoparticle group when compared with the free berberine group. The delayed Tmax in the berberine nanoparticle group confirmed the sustained berberine release in vivo, which is consistent with the in vitro release profile results.
TABLE 2
| Parameter | Unit | Berberine nanoparticles | Free berberine | p-value |
|---|---|---|---|---|
| Dose | mg/kg | 50 | 50 | |
| Cmax | ng/mL | 399 ± 26 | 141 ± 35 | <0.0001 |
| Tmax | h | 2 | 0.5 | |
| AUC | ng/h/mL | 2,926 ± 75 | 692 ± 74 | <0.0001 |
| BAR | - | - | 2.8-fold |
Pharmacokinetic parameters of berberine-loaded chitosan/SLN nanoparticles and free berberine in rats after a single oral dose administration.
AUC, area under the berberine plasma concentration-time curve; BAR, the relative bioavailability of oral administration of berberine-loaded chitosan/SLN nanoparticles compared with the pure berberine suspension; Cmax, the maximum plasma concentration of berberine after oral administration; Tmax, the time to maximum plasma concentration of berberine.
Berberine nanoparticles inhibit body weight reduction in the GDM rats
The body weight reduction is a major diabetic complication, and is known as an important parameter to estimate diabetes. The body weight of rats before pregnancy (GD0) and after 2 weeks of treatment (GD18) was measured (Figure 2A). The body weight showed no significant difference between all groups of rats before pregnancy at GD0. All group rats showed significant body weight elevation (p < 0.0001) at GD18 compared to the initial body weight at GD0. The GDM rats demonstrated a significant body weight reduction (p < 0.0001) compared to the NPC group and treatment groups at GD18. GDM rats treated with the low and high doses of berberine nano-formulations (LBN and HBN groups) and of free-berberine (LBF and HBF) demonstrated a significant dose-dependent body weight elevation (p < 0.0001) by (65.7 ± 3.6 g and 79.6 ± 4.6 g) and (40.3 ± 3.3 g and 42.6 ± 3.3 g), respectively, compared with the GDM group at GD18. Of note, the body weight showed no significant (p = 0.7) difference between the HBN group (295 ± 10.5 g) and the metformin-treated (STD) group (301.6 ± 11 g) at GD18. In addition, there was a significant (p = 0.01), but low difference (19 ± 4.6 g) between the body weight of the HBN group (295 ± 10.5 g) and the NPC group (314 ± 6 g) at GD18.
FIGURE 2
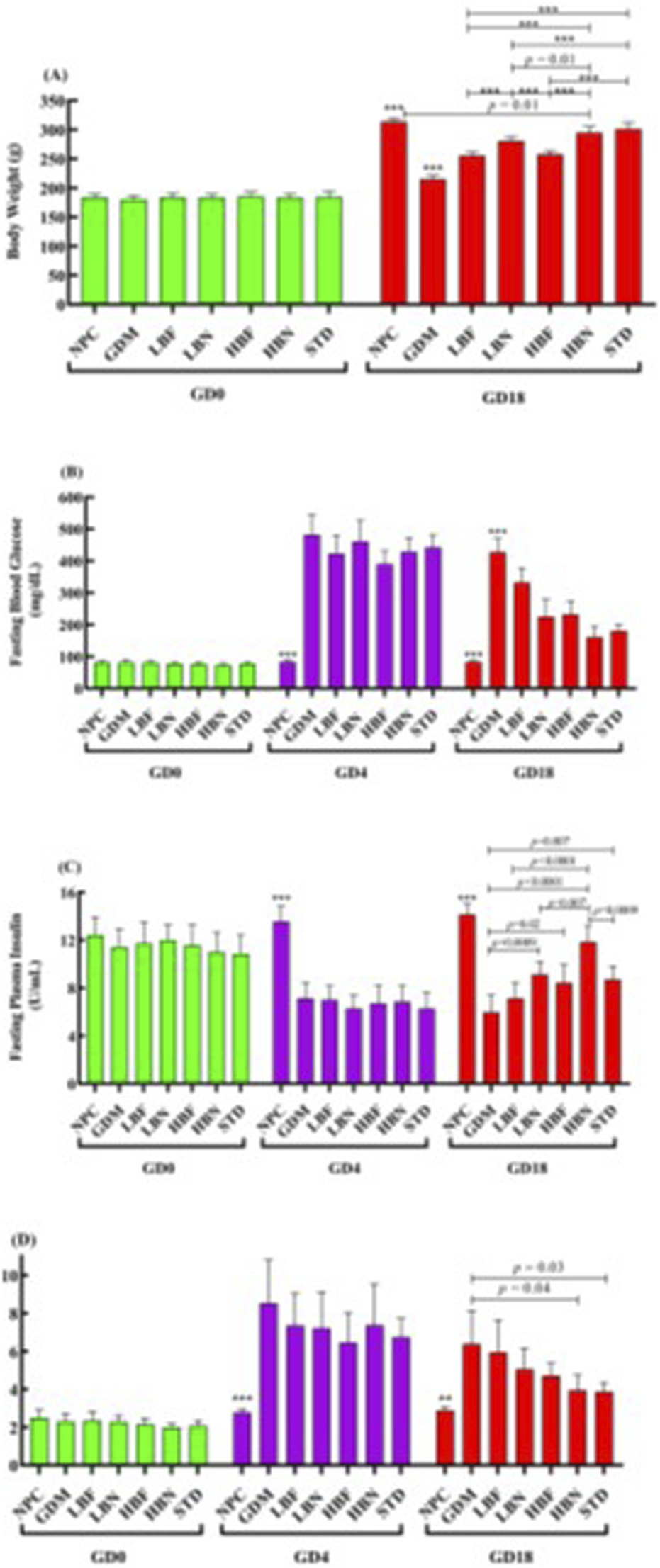
Body weight of rats before pregnancy (GD0) and after 2 weeks treatment (GD18) in the normal pregnant control (NPC) group, gestational diabetes mellitus (GDM) group, GDM rats received a low dose of berberine nanoparticles (the LBN group, 25 mg/kg/day), GDM rats received a high dose of berberine nanoparticles (the HBN group, 50 mg/kg/day), GDM rats received a low dose of free-berberine (the LBF group, 25 mg/kg/day), GDM rats received a high dose of free-berberine (the HBF group, 50 mg/kg/day), and the standard (STD) group including GDM rats received metformin (200 mg/kg/day) (A). Levels of fasting blood glucose (B) and fasting plasma insulin (C) in NPC, GDM, LBN, HBN, LBF, HBF, and STD groups at GD0, 4 days after STZ injection (GD4), and GD18 (D). The scores of Homeostasis Model of Insulin Resistance (HOMA-IR) in the NPC, GDM, LBN, HBN, LBF, HBF, and STD groups at GD0, GD4, and GD18. Data are represented as the mean ± SD (n = 7). ** and *** indicate p < 0.001 and p < 0.0001, respectively, for the NPC group or the GDM group when compared with other groups.
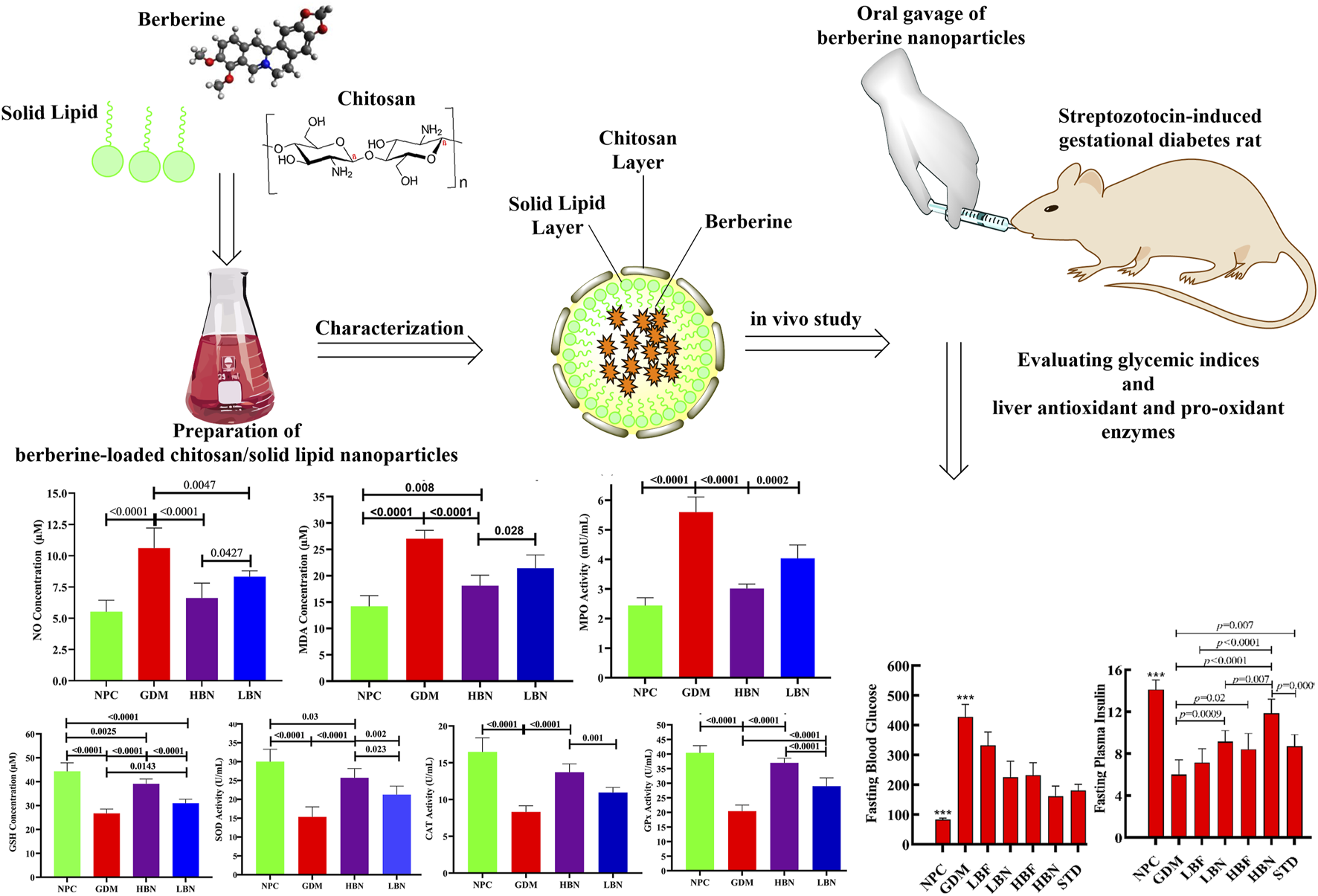
Berberine nanoparticles improve fasting glucose and insulin in the GDM rats
The changes in the FBG levels are shown in Figure 2B. Four days after STZ injection (GD4), the FBG assessing showed that STZ-injected pregnant rats subjected to a remarkable (p < 0.0001) hyperglycemia (482 ± 63 mg/dL) at GD4 as compared with the NPC group (83 ± 5 mg/dL), verifying STZ-induced GDM. The FBG concentration was remarkably elevated in the GDM group at GD18 as compared with the NPC group (p < 0.0001). However, the FBG level in the LBN (225 ± 54 mg/dL), HBN (161 ± 35 mg/dL), LBF (332 ± 45 mg/dL), and HBF (232 ± 42 mg/dL) groups was remarkably reduced by 203 ± 26 mg/dL (p < 0.0001), 267 ± 20 mg/dL (p < 0.0001), 95 ± 23 mg/dL (p = 0.007), and 195 ± 22 mg/dL (p < 0.0001), respectively, as compared with the GMD group at GD18.
The level of fasting plasma insulin was significantly improved in the GDM rats treated with berberine nano-formulation and free-berberine at GD18 (Figure 2C). The fasting insulin level in the NPC group remained stable at GD0, GD4, and GD18, and it was significantly (p < 0.0001) decreased in the GDM group at GD4 and GD18 by 6.5 ± 0.7 U/mL and 8.2 ± 0.7 U/mL, respectively, as compared with the NPC group. Of note, the level of fasting insulin in the LBN (9.2 ± 1 U/mL), HBN (11.8 ± 1.3 U/mL), and HBF (8.5 ± 1.5 U/mL) groups was significantly increased by 3.14 ± 0.67 U/mL (p = 0.0009), 5.9 ± 0.7 U/mL (p < 0.0001), and 2.5 ± 0.8 U/mL (p = 0.02), respectively, at GD18 as compared with the GMD group (6.0 ± 1.4 U/mL). Fasting level of insulin (7.1 ± 1.3 U/mL) was non-significantly increased by 1.14 ± 0.7 U/mL (p = 0.47) in the LBF group at GD18 as compared with the GMD group.
As shown in Figure 2D, the HOMA-IR score was significantly elevated by 2-fold (p = 0.002) and 1.2-fold (p = 0.007) in the GDM group at GD4 and GD18, respectively, as compared with the NPC group, whereas it was reduced significantly in the HBN group by 0.38-fold (p = 0.04) and non-significantly in the LBN, LBF, and HBF groups by 0.2-fold (p = 0.4), 0.07-fold (p = 0.1), and 0.26-fold (p = 0.17), respectively, at GD18 as compared with the GMD group. Furthermore, treatment with the high-dose berberine nano-formulation could improve the FBG, fasting plasma insulin, and HOMA-IR score in the HBN group close to those in the STD group treated with metformin.
Berberine nanoparticles improve glucose tolerance in the GDM rats
To determine glucose tolerance in the treated GDM rats, an OGTT over 120 min was carried out after 2 weeks of treatment (GD18). 30 min after oral gavage of glucose, a remarkable elevation in the level of blood glucose was detected in the GDM rats compared with NPC rats, indicating a significantly impaired tolerance to exogenously administered glucose. Glucose tolerance ability was remarkably enhanced in the treated GDM rats compared to the untreated GDM rats. A significant reduction in glucose levels was detected after 30 min toward baseline at time 120 in the treated GDM rats (LBN and HBN groups), whereas a consistently high concentration of glucose was found during 30–120 min in the GDM rats. In addition, the blood level of glucose slowly decreased after 30 min in the LBF and HBF groups; however, it did not reach the baseline level at 120 min (Figure 3A).
FIGURE 3
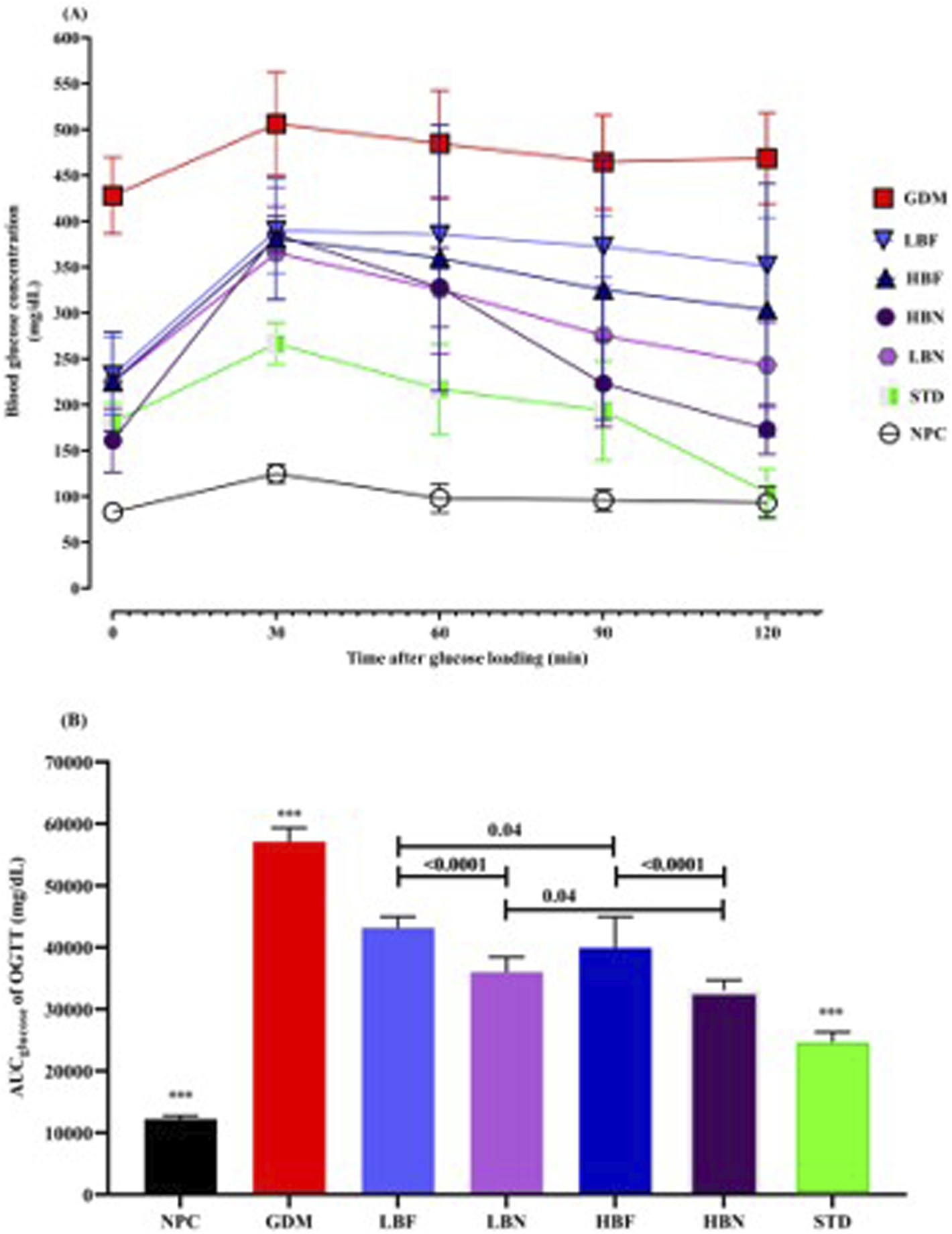
Oral glucose tolerance test (A) and corresponding areas under the glucose curve (AUCglucose) (B) over 120 min after the oral glucose was fed in the NPC, GDM, LBN, HBN, LBF, HBF, and STD groups at GD18. Data are represented as the mean ± SD (n = 7). *** indicates p < 0.0001 for NPC group, GDM group, or STD group when compared with treatment groups.
AUCglucose scores over 120 min of treated GDM rats and GDM rats were remarkably (p < 0.0001) higher than NPC rats. Notably, analyzing AUCglucose scores indicated that blood levels of glucose in the different groups of treated GDM rats, including the LBN, HBN, LBF, and HBF groups, were significantly (p < 0.0001) reduced up to 37%, 42%, 24%, and 30%, respectively, compared with the GDM group. As the positive control, metformin reduced AUC values up to 57% in the STD group (Figure 3B).
Berberine nanoparticles improve insulin response in the GDM rats
To evaluate insulin response in the treated GDM rats, the insulin challenge (0.8 U/kg, i.p.) was conducted at GD19. The blood glucose levels and AUCglucose were significantly (p < 0.0001) higher at various time points after the insulin injection in the GDM group compared with the NPC group, showing a remarkably impaired insulin response in the GDM rats. The insulin response ability was significantly enhanced in the treated GDM rats compared to GDM rats. The blood levels of glucose and AUCglucose were lower during ITT in treated GDM rats than in GDM rats (Figure 4). As compared to the GDM group, AUCglucose values in different groups of treated GDM rats, including the LBN, HBN, LBF, and HBF groups, demonstrated significant (p < 0.0001) reduction by 52%, 66%, 25%, and 44%, respectively. In addition, AUCglucose values were found to be significantly decreased by 73% (p < 0.0001) in the STD group treated with metformin, when compared with the GDM group (Figure 4B).
FIGURE 4
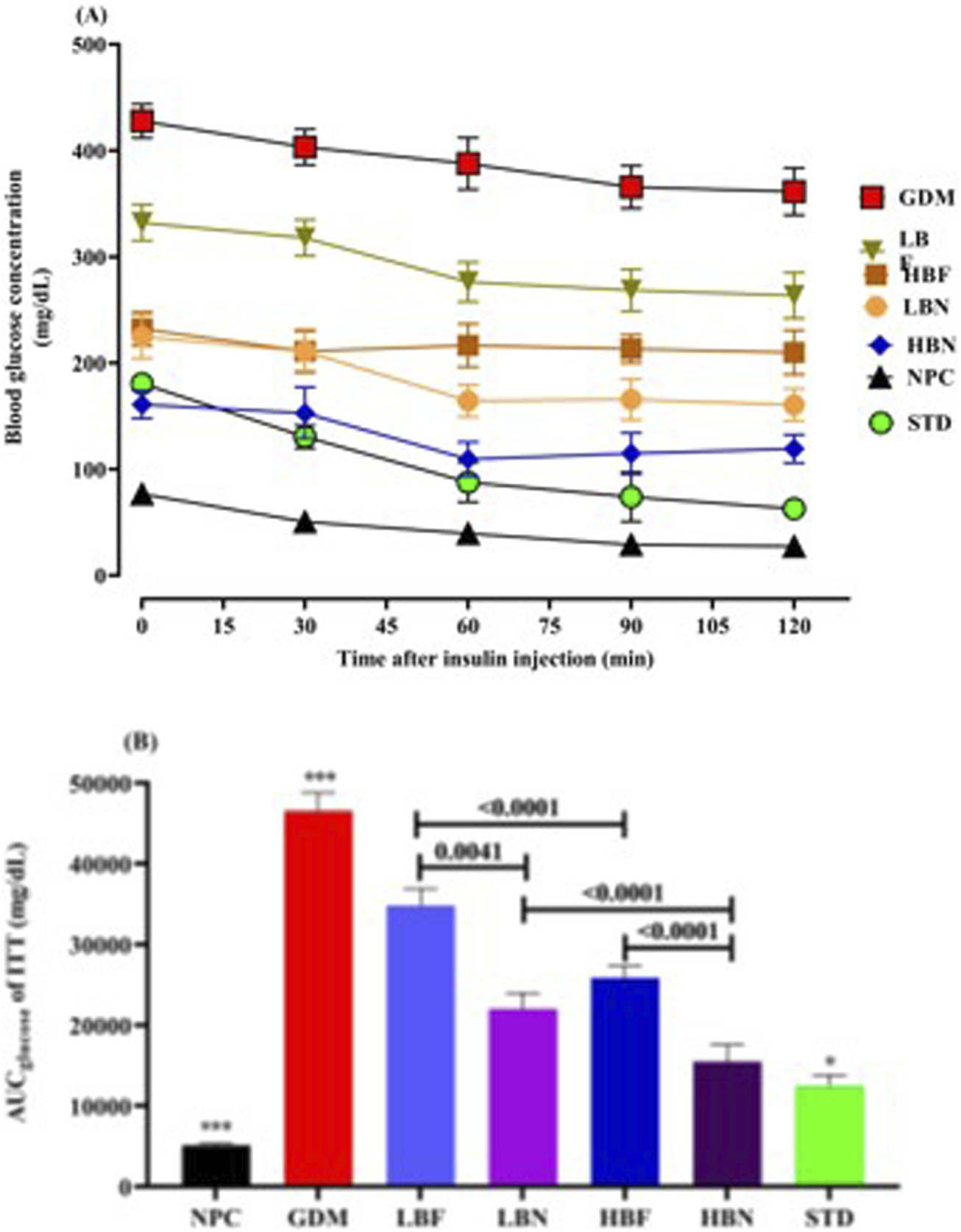
The Insulin tolerance test (A) and corresponding areas under the glucose curve (AUCglucose) (B) over 120 min after the insulin injection in the NPC, GDM, LBN, HBN, LBF, HBF, and STD groups at GD18. Data are represented as the mean ± SD (n = 7). *** indicates p < 0.0001 for NPC group or GDM group when compared with treatment groups. * indicates p < 0.03 for the STD group when compared with the treatment groups.
Berberine nanoparticles improve the hepatic antioxidant capacity in the GDM rats
To determine the impact of berberine nanoparticles on the antioxidant status in GDM rats, the concentration of GSH and the activity of antioxidant enzymes GPx, CAT, and SOD were measured in hepatic tissues at the end of the study. As demonstrated in the Figure 5, the level of GSH (26.72 ± 1.86 µM) and the activity of SOD (15.35 ± 3.3 U/mL), CAT (6.93 ± 0.84 U/mL), and GPx (20.43 ± 2.1 U/mL) were significantly decreased by – 38-fold (p < 0.0001), – 0.47-fold (p < 0.0001), – 0.51-fold (p < 0.0001), and – 0.49-fold (p < 0.0001), respectively, in the GDM group compared with those (44.34 ± 3.57 µM, 30.01 ± 2.64 U/mL, 16.47 ± 1.92 U/mL, and 40.41 ± 2.49, respectively) in the NPC group. However, when the GDM rats were treated with the high and low doses of berberine nano-formulations (HBN and LBN groups), decreased values of GSH, SOD, CAT, and GPx were significantly and dose-dependently elevated by (0.46-fold (p < 0.0001) and 0.16-fold (p = 0.01)), (0.67-fold (p < 0.0001) and 0.38-fold (p = 0.002)), (0.65-fold (p < 0.0001) and 0.32-fold (p = 0.003)), and (0.81-fold (p < 0.0001) and 0.42-fold (p < 0.0001)), respectively, in comparison with the GDM group. Additionally, a low but significant difference was found between the mean values of antioxidant parameters in treatment (HBN and LBN) groups and the NPC group.
FIGURE 5
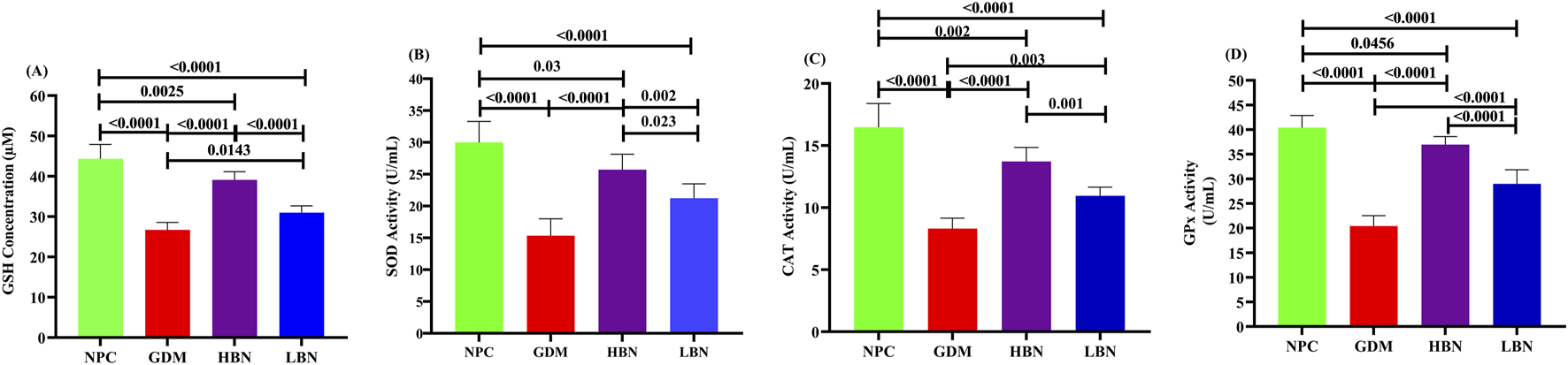
The level of GSH (A) and the activity of antioxidant enzymes, including SOD (B), CAT (C), and GPx (D) in the NPC, GDM, LBN, and HBN groups at GD18. Data are represented as the mean ± SD (n = 7).
Berberine nanoparticles ameliorate the hepatic pro-oxidant load in the GDM rats
To further evaluate the effect of the high and low doses of berberine nanoformulations on oxidative stress, the concentrations of NO and MDA, as well as the activity of pro-oxidant enzyme MPO, were measured in the liver tissue at GD19. As shown in Figure 6, the levels of NO (10.61 ± 1.61 µM) and MDA (27.01 ± 1.6 µM) as well as the activity of MPO (5.6 ± 0.51 mU/mL) were significantly increased by 0.92-fold (p < 0.0001), 0.9-fold (p < 0.0001), 1.3-fold (p < 0.0001), and – 0.49-fold (p < 0.0001), respectively, in the GDM group compared with those (5.52 ± 0.9 µM, 14.2 ± 2 μM, and 2.44 ± 0.26 mU/mL, respectively) in the NPC group. However, when the GDM rats were treated with the high and low doses of berberine nano-formulations, the increased values of NO, MDA, and MPA were significantly and dose-dependently reduced by (– 0.38-fold (p < 0.0001) and – 0.21-fold (p = 0.004)), (– 0.33-fold (p < 0.0001) and – 0.2-fold (p = 0.0002)), and (– 0.46-fold (p < 0.0001) and – 0.28-fold (p < 0.0001)), in the HBN and LBN groups, respectively, in comparison with the GDM group. Additionally, a significant but low difference (p < 0.05) were detected between mean values of pro-oxidant parameters in the treatment (HBN and LBN) groups and the NPC group.
FIGURE 6
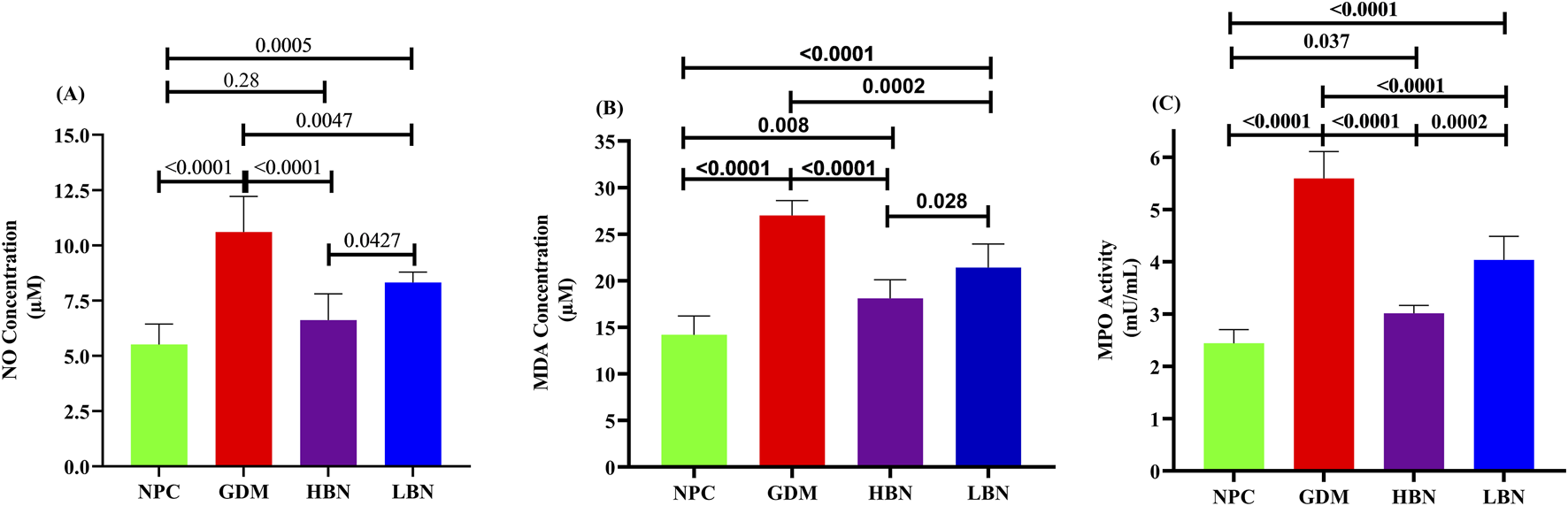
The liver levels of NO (A) and MDA (B), as well as the activity of pro-oxidant enzyme MPO (C) in the NPC, GDM, LBN, and HBN groups at GD18. Data are represented as the mean ± SD (n = 7).
Discussion
The results from our study indicate that entrapment in chitosan-coated SLN nanoparticles can significantly improve the bioavailability and hypoglycemic effects of berberine in STZ-provoked GDM rats.
Berberine’s ameliorative impacts on the glycemic indices in diabetes mellitus have been well-documented by various preclinical [63–66] and clinical studies [67–71]. There is also growing preclinical evidence indicating the potential of berberine in the GDM treatment. Yang et al. showed that oral gavage of berberine (25, 50, and 100 mg/kg/day from the day before pregnancy till 21st day of pregnancy) could dose-dependently improve body and fetal weight, placental weight, glucose tolerance, and insulin response in the STZ-induced GDM rats [25]. Consistently, Li et al. showed that orally administrated berberine (100 mg/kg/day, gavaged from the 7th to 20th day of pregnancy) could significantly improve maternal insulin response and body weight, placental and fetal weight, as well as the number of dead and absorptive fetuses in the high fat diet-induced GDM rats compared with the GDM rats without berberine treatment [26]. Similarly, other preclinical studies also reported the anti-diabetic impacts of berberine in GDM [21, 27, 28]. However, the poor hydrophilicity and bioavailability of berberine limit therapeutic efficiency after oral administration. Of note, berberine has to be repeatedly used at high doses (0.9–1.5 g/day) once prescribed to patients with diabetes [23]. Though berberine at high doses effectively ameliorates hyperglycemia, it leads to considerable gastrointestinal adverse impacts that limit its clinical use. Thus, improving the oral bioavailability of berberine not only elevates its anti-hyperglycemic impact but also decreases gastrointestinal adverse impacts. To improve berberine bioavailability, numerous investigations have developed berberine nanoformulations and showed their improved effectiveness for treating various diseases, such as diabetes [72, 73]. Based on our current knowledge, there is no published report showing the potential of berberine nanoformulations in GDM treatment. Here, we first studied the anti-hyperglycemic effects of berberine-loaded chitosan/SLN nanoparticles in the STZ-induced GDM rats. STZ induces a diabetes model identified by pancreatic β-cells’ damage, causing insulin deficiency and consequent hyperglycemia and body weight reduction [74]. We found a significantly increased blood glucose concentration and body weight loss in STZ-administered pregnant rats (GDM group), when compared with normal pregnant rats (NPC group). Two weeks’ daily oral gavage of free-berberine (50 and 100 mg/kg/day) could dose-dependently reverse the STZ-induced insulin deficiency, hyperglycemia, and body weight loss in the GDM rats, which were consistent with the above-mentioned studies [21, 25–28]. Of note, we indicated that the ameliorating effects of berberine on glycemic indices and body weight loss were significantly improved when encapsulated in chitosan-coated SLN nanoparticles. The low (25 mg/kg/day) and high (50 mg/kg/day) doses of berberine nanoparticles were found to significantly increase the reduction of FBG levels by 2.1-fold and 1.4-fold in the GDM rats, respectively, when compared with the equal doses of free-berberine. As shown by OGTT analysis, reduction of the blood glucose levels at the given times after glucose challenge in free-berberine- and berberine nanoparticle-treated GDM rats showed a 0.54-fold and 0.4-fold higher glucose tolerance in the GDM rats treated by the low and high doses of berberine nanoparticles, respectively, than those treated by free-berberine. The present findings are similar to other studies showing the enhanced efficacy of other berberine nanoformulations in reducing the elevated levels of FBG in STZ-induced diabetic rodents [75, 76]. Such a reduction of blood glucose levels might be attributed to elevated peripheral utilization of glucose. Our results from evaluating fasting insulin suggest that this can be due to the inducing effect of berberine on insulin secretion by pancreatic cells. Notably, the level of fasting plasma insulin was remarkably reduced in the untreated STZ-induced GDM rats compared with NPC rats, showing impaired insulin secretion. Interestingly, berberine nanoparticle-treated GDM rats demonstrated a significant elevation in fasting insulin levels, where a 2.75-fold and 2.4-fold elevation in fasting insulin was found in GDM rats treated by berberine nanoparticles at the low and high doses, respectively, in comparison with those treated by free berberine. However, low-dose free berberine showed no significant elevation in fasting insulin levels in GDM rats, whereas the high-dose could exert a significant improving effect. Mechanistically, berberine can enhance peripheral usage of glucose, at least in part, through promoting insulin production via pancreatic cells. Zhou et al. reported that berberine can significantly elevate islet area, the number of β-cells, the insulin secretion, and the ratio of pancreas to body weight in diabetic rats [77]. Leng et al. found that berberine dose-dependently induces insulin secretion in HIT-T15 cells and mouse islets [78]. Consistently, Wang et al. showed that berberine causes an insulinotropic effect in rat islets [79]. It has also been found that berberine can inhibit β-cell apoptosis and induce insulin release [80, 81]. Mechanistically, berberine has been shown to induce insulin secretion by elevating the levels of the incretin hormone glucagon-like peptide-1 (GLP-1) that participates in the survival of pancreatic cells. GLP-1, by activating adenylate cyclase that converts ATP into cyclic adenosine monophosphate (cAMP), triggers the epac protein and PKA signaling, leading to an elevation in the intracellular level of Ca2+. This promotes the translocation and secretion of insulin granules [82–85].
Besides increasing insulin secretion, results from the ITT’s insulin challenge and the HOMA-IR scores suggest that berberine might increase peripheral glucose uptake in STZ-induced GDM rats through enhancing the sensitivity/response of the body’s cells to the insulin effect. Notably, ITT assessment revealed that, after insulin administration, untreated GDM rats were unable to significantly reduce the blood glucose, indicating that GDM rats suffered from a peripheral response deficiency to insulin and thus were unable to utilize exogenously injected insulin to drop the increased levels of blood glucose. However, free berberine and berberine nanoparticles remarkably decreased blood glucose in GDM rats after insulin injection, while 1.1-fold and 0.5-fold reductions were detected in GDM rats that received berberine nanoparticles at low and high doses, respectively, in comparison with those treated by free berberine. Supporting this, the HOMA-IR scores revealed that insulin resistance was significantly reduced by 2.85-fold and 1.5-fold in GDM rats treated with the low and high doses of berberine nanoparticles, respectively, when compared with free-berberine, further verifying an improved response of the body’s cells to the insulin effect in the berberine nanoparticle-treated GDM rats.
During the insulin resistance condition, there is a dysregulation in the insulin response signaling pathway, the Protein Kinase B (Akt)/Phosphoinositide 3-kinase (PI3K)/Insulin receptor substrate 1 (IRS-1). In improving insulin resistance, mechanistically, berberine can induce insulin response by activating Akt through promoting the 5′-adenosine monophosphate-activated protein kinase (AMPK) signaling as an energy-sensing pathway that is activated/inactivated in accordance with the cellular AMP/ATP ratio [82, 86, 87]. Berberine induces AMPK by promoting phosphorylation of Thr172 on the α subunit of AMPK [88–90], as well as by elevating the ratio of cellular AMP/ATP through suppressing the generation of the mitochondrial ATP [91, 92]. Berberine by AMPK can activate Akt that induces the expression and translocation of glucose transporter type-4 (GLUT4) to the cellular membrane, thus, glucose can be uptaken and utilized by the cell, causing increased glucose uptake and usage by insulin-resistant cells [82].
Furthermore, weight reduction is the main property of human T2DM and STZ-induced diabetes because of degradation of structural proteins and muscle injury, as well as worsened lipolysis and increased lipid peroxidation, which are complications of insulin deficiency [93]. Here, we found that berberine nanoformulation and free berberine treatment could significantly inhibit the body weight reduction in the STZ-induced GDM rats, where a 1.6-fold and 1.9-fold elevation of the body weight was found in the GDM rats treated by low and high doses of berberine nanoparticles, respectively, when compared with those treated by the equal doses of free berberine. Similarly, Yang et al. reported the preventive impact of berberine against body weight loss in the STZ-induced GDM rats [25].
Increased oxidative stress, resulting from an imbalance of free radicals producing and scavenging, is another common characteristic in the diabetes onset and development [94–96]. The results of the present investigation showed that berberine nanoparticles can significantly enhance hepatic antioxidant capacity in the STZ-induced GDM rats through increasing the concentration of GSH and the activity of antioxidant enzymes GPx, CAT, and SOD. Further, berberine nanoparticles could significantly reverse the increased levels of NO and MDA, as well as the enhanced activity of pro-oxidant enzyme MPO in STZ-induced GDM rats. Of note, the difference between the berberine nanoparticle-treated GDM rats (HBN/LBN groups) and the NPC rats was found to be small but sufficient. Mechanistically, berberine has been found to upregulate uncoupling protein 2 (UCP2) as a mitochondrial inner membrane protein that has a significantly negative association with ROS generation and oxidative stress [82].
The better protection against diabetes in STZ-induced GDM rats by berberine-loaded chitosan/SLN nanoparticle treatment than free berberine treatment can be attributed to a higher oral bioavailability of berberine achieved by chitosan-coated SLN formulation. After entrapment in chitosan/SLN nanoparticles, berberine showed an elevated level of the plasma peak, a postponed peak time, and an increased AUC in rats, confirming an improved oral bioavailability of berberine. The improved bioavailability of berberine can be attributed to the physicochemical features of berberine-loaded chitosan/SLN nanoparticles, as discussed in the following. The in vitro stability and drug release assessment demonstrated that berberine nanoparticles are stable in the SGF condition and show a sustained release profile in the SIF condition. Further, the chitosan moiety provides a hydrophilic surface layer that enables nanoparticles to form strong hydrogen bonds to the intestinal mucosal surface and, thus, enhances the mucoadhesion. The supporting investigations reported the enhanced intestinal uptake of compounds entrapped in chitosan-coated SLN nanoparticles, which was found to be attributed to hydrophilic and ionic interactions [37, 39]. These nanoparticles also showed a positive surface charge that causes an easy attachment to the intestinal cell membrane with the negative charge, leading to an enhanced uptake by intestinal cells [97]. Moreover, particle size can directly influence the cellular uptake of nanoparticles [98]. Berberine-loaded chitosan/SLN nanoparticles demonstrated a nanosize that facilitates an efficient cellular uptake. A phenomenon known as membrane wrapping, which demonstrates how a membrane may enclose a molecule, can explain the size-dependent uptake of nanoparticles. The nano size of berberine-loaded chitosan/SLN particles is also suitable for invisibility in the reticuloendothelial system and long-term circulation in the bloodstream [98, 99].
Conclusion
We synthesized chitosan-coated SLN nanoparticles to deliver berberine and evaluate their antidiabetic impacts in STZ-provoked GDM rats. The stable and nano-sized particle, high EE%, good in vivo stability, and sustained berberine release features are shown by berberine-loaded chitosan/SLN nanoparticles. Compared with free berberine treatment, berberine nanoparticle treatment could provide a significantly higher oral bioavailability of berberine in experimental rats. Chitosan-coated SLN nanoparticles can significantly enhance the protective effect of berberine against hyperglycemia in the STZ-induced GDM rats. Notably, the improved bioavailability of berberine-loaded chitosan/SLN nanoparticles is a possible reason supporting the improved therapeutic impact of berberine in the STZ-induced GDM rats. In conclusion, chitosan-coated SLN nanoparticles provide a suitable delivery system to enhance the oral bioavailability of berberine and, thus, improve its pharmacological impacts.
Statements
Data availability statement
The data used to support the findings of this study are available from the corresponding author upon request.
Ethics statement
The animal study was approved by Zhinanzhen Biology Ethics Committee, No.: A2025000127. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
The authors declare that financial support was received for the research and/or publication of this article. The authors would like to acknowledge the funding from the Ongoing Research Funding Program, (ORF-2026-371), King Saud University, Riyadh, Saudi Arabia.
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
References
1.
LeeJLeeNKMoonJH. Gestational diabetes mellitus: mechanisms underlying maternal and fetal complications. Endocrinol Metab (2025) 40:10–25. 10.3803/enm.2024.2264
2.
Quintanilla RodriguezBSVadakekutESMahdyH. Gestational diabetes. Treasure Island (FL): StatPearls Publishing (2024).
3.
Ruiz-MartínezMLGómez-DíazRAValdez GonzálezALÁngeles MejíaSMondragón GonzálezRDíaz FloresMet alAssociation of oxidative stress markers with incident hyperglycemia in gestational diabetes mellitus in an educational intervention. Nutrients (2025) 17:680. 10.3390/nu17040680
4.
American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in Diabetes-2018. Diabetes Care (2018) 41:S13–s27. 10.2337/dc18-s002
5.
BobadePSGanorkarSBBaviskarPNPatilNBShindeDBShirkhedkarAAet alPharmaceutical analysis of berberine: an update. Separation and Purif Rev (2024) 54:1–10. 10.1080/15422119.2024.2423403
6.
ShenPJiaoYMiaoLChenJMomtazi‐BorojeniAA. Immunomodulatory effects of berberine on the inflamed joint reveal new therapeutic targets for rheumatoid arthritis management. J Cell Mol Med (2020) 24:12234–45. 10.1111/jcmm.15803
7.
FatahianAHaftcheshmehSMAzhdariSFarshchiHKNikfarBMomtazi-BorojeniAA. Promising anti-atherosclerotic effect of berberine: evidence from in vitro, and clinical studies. Rev Physiol Biochem Pharmacol (2020) 178:83–110. 10.1007/112_2020_42
8.
AyatiSHFazeliBMomtazi-BorojeniAACiceroAFPirroMSahebkarA. Regulatory effects of berberine on microRNome in cancer and other conditions. Crit Reviews Oncology/hematology (2017) 116:147–58. 10.1016/j.critrevonc.2017.05.008
9.
MortazaviHNikfarBEsmaeiliS-ARafieeniaFSaburiEChaichianSet alPotential cytotoxic and anti-metastatic effects of berberine on gynaecological cancers with drug-associated resistance. Eur Journal Medicinal Chemistry (2020) 187:111951. 10.1016/j.ejmech.2019.111951
10.
Mohammadian HaftcheshmehSMomtazi‐BorojeniAA. Berberine as a promising natural compound for the treatment of periodontal disease: a focus on anti‐inflammatory properties. J Cell Mol Med (2021) 25:11333–7. 10.1111/jcmm.17019
11.
LanJZhaoYDongFYanZZhengWFanJet alMeta-analysis of the effect and safety of berberine in the treatment of type 2 diabetes mellitus, hyperlipemia and hypertension. J Ethnopharmacology (2015) 161:69–81. 10.1016/j.jep.2014.09.049
12.
MaXChenZWangLWangGWangZDongXet alThe pathogenesis of diabetes mellitus by oxidative stress and inflammation: its inhibition by berberine. Front Pharmacol (2018) 9:782. 10.3389/fphar.2018.00782
13.
EhteshamfarSMAkhbariMAfshariJTSeyediMNikfarBShapouri‐MoghaddamAet alAnti‐inflammatory and immune‐modulatory impacts of berberine on activation of autoreactive T cells in autoimmune inflammation. J Cellular Molecular Medicine (2020) 24:13573–88. 10.1111/jcmm.16049
14.
MoCWangLZhangJNumazawaSTangHTangXet alThe crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Antioxid and Redox Signaling (2014) 20:574–88. 10.1089/ars.2012.5116
15.
HaftcheshmehSMAbediMMashayekhiKMousaviMJNavashenaqJGMohammadiAet alBerberine as a natural modulator of inflammatory signaling pathways in the immune system: focus on NF‐κB, JAK/STAT, and MAPK signaling pathways. Phytotherapy Res (2022) 36:1216–30. 10.1002/ptr.7407
16.
ChenQ-MXieMZ. Studies on the hypoglycemic effect of Coptis chinensis and berberine. Yao Xue Xue Bao= Acta Pharmaceutica Sinica (1986) 21:401–6.
17.
NiYX. Therapeutic effect of berberine on 60 patients with type II diabetes mellitus and experimental research. Zhong Xi Yi Jie He Za Zhi= Chin J Mod Dev Traditional Med (1988) 8:711–3.
18.
XieWSuFWangGPengZXuYZhangYet alGlucose-lowering effect of berberine on type 2 diabetes: a systematic review and meta-analysis. Front Pharmacol (2022) 13:4734. 10.3389/fphar.2022.1015045
19.
LiangYXuXYinMZhangYHuangLChenRet alEffects of berberine on blood glucose in patients with type 2 diabetes mellitus: a systematic literature review and a meta-analysis. Endocr Journal (2019) 66:51–63. 10.1507/endocrj.ej18-0109
20.
WeiX-cZhuL-qWangC-g. Efficacy and safety of berberine in patients with type 2 diabetes mellitus: a meta-analysis. Chin Herbal Medicines (2015) 7:344–53. 10.1016/s1674-6384(15)60063-6
21.
ColeLKZhangMChenLSparagnaGCVandelMXiangBet alSupplemental berberine in a high-fat diet reduces adiposity and cardiac dysfunction in offspring of mouse dams with gestational diabetes mellitus. The J Nutr (2021) 151:892–901. 10.1093/jn/nxaa408
22.
ColeLKSparagnaGCVandelMXiangBDolinskyVWHatchGM. Berberine elevates cardiolipin in heart of offspring from mouse dams with high fat diet-induced gestational diabetes mellitus. Scientific Rep (2021) 11:15770. 10.1038/s41598-021-95353-4
23.
YinJXingHYeJ. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism (2008) 57:712–7. 10.1016/j.metabol.2008.01.013
24.
ZhangHWeiJXueRWuJ-DZhaoWWangZ-Zet alBerberine lowers blood glucose in type 2 diabetes mellitus patients through increasing insulin receptor expression. Metabolism (2010) 59:285–92. 10.1016/j.metabol.2009.07.029
25.
YangXYangCLuW. Berberine suppresses gestational diabetes in streptozotocin-induced diabetes mellitus rats by suppression of inflammatory mediators. Indian J Pharm Education Res (2023) 57:423–31. 10.5530/ijper.57.2.53
26.
LiALinCXieFJinMLinF. Berberine ameliorates insulin resistance by inhibiting IKK/NF-κB, JNK, and IRS-1/AKT signaling pathway in liver of gestational diabetes mellitus rats. Metab Syndr Relat Disord (2022) 20:480–8. 10.1089/met.2022.0017
27.
FengLINJieWUXiaoWYin-pingHJingZ. Experimental study of berberine improving insulin resistance in gestational diabetes mellitus rat model and its possible mechanisms. Chinese Journal of General Practice (2019) 17:1647–51. 10.16766/j.cnki.issn.1674-4152.001019
28.
WangYGongWLvSQuHHeY. Berberine improves insulin resistance in adipocyte models by regulating the methylation of hypoxia-inducible factor-3α. Biosci Rep (2019) 39:BSR20192059. 10.1042/bsr20192059
29.
HanYXiangYShiYTangXPanLGaoJet alPharmacokinetics and pharmacological activities of berberine in diabetes mellitus treatment. Evid Based Complement Altern Med (2021) 2021:1–15. 10.1155/2021/9987097
30.
XuXYiHWuJKuangTZhangJLiQet alTherapeutic effect of berberine on metabolic diseases: both pharmacological data and clinical evidence. Biomed and Pharmacother (2021) 133:110984. 10.1016/j.biopha.2020.110984
31.
HuaWDingLChenYGongBHeJXuG. Determination of berberine in human plasma by liquid chromatography–electrospray ionization–mass spectrometry. J Pharmaceutical Biomedical Analysis (2007) 44:931–7. 10.1016/j.jpba.2007.03.022
32.
ZuoFNakamuraNAkaoTHattoriM. Pharmacokinetics of berberine and its main metabolites in conventional and pseudo germ-free rats determined by liquid chromatography/Ion trap mass spectrometry. Drug Metab Disposition (2006) 34:2064–72. 10.1124/dmd.106.011361
33.
LiuY-THaoH-PXieH-GLaiLWangQLiuC-Xet alExtensive intestinal first-pass elimination and predominant hepatic distribution of berberine explain its low plasma levels in rats. Drug Metab Disposition (2010) 38:1779–84. 10.1124/dmd.110.033936
34.
LiuC-SZhengY-RZhangY-FLongX-Y. Research progress on berberine with a special focus on its oral bioavailability. Fitoterapia (2016) 109:274–82. 10.1016/j.fitote.2016.02.001
35.
ChenCFanTJinYZhouZYangYZhuXet alOrally delivered salmon calcitonin-loaded solid lipid nanoparticles prepared by micelle–double emulsion method via the combined use of different solid lipids. Nanomedicine (2013) 8:1085–100. 10.2217/nnm.12.141
36.
GeorgeMAbrahamTE. Polyionic hydrocolloids for the intestinal delivery of protein drugs: alginate and chitosan—a review. J Controlled Release (2006) 114:1–14. 10.1016/j.jconrel.2006.04.017
37.
LuoYTengZLiYWangQ. Solid lipid nanoparticles for oral drug delivery: Chitosan coating improves stability, controlled delivery, mucoadhesion and cellular uptake. Carbohydr Polymers (2015) 122:221–9. 10.1016/j.carbpol.2014.12.084
38.
SogiasIAWilliamsACKhutoryanskiyVV. Why is chitosan mucoadhesive?Biomacromolecules (2008) 9:1837–42. 10.1021/bm800276d
39.
FontePNogueiraTGehmCFerreiraDSarmentoB. Chitosan-coated solid lipid nanoparticles enhance the oral absorption of insulin. Drug Deliv Translational Res (2011) 1:299–308. 10.1007/s13346-011-0023-5
40.
NairRKumarACPriyaVKYadavCMRajuPY. Formulation and evaluation of chitosan solid lipid nanoparticles of carbamazepine. Lipids Health Disease (2012) 11:1–8. 10.1186/1476-511x-11-72
41.
RidolfiDMMarcatoPDJustoGZCordiLMachadoDDuránN. Chitosan-solid lipid nanoparticles as carriers for topical delivery of tretinoin. Colloids Surf B: Biointerfaces (2012) 93:36–40. 10.1016/j.colsurfb.2011.11.051
42.
HuF-QJiangS-PDuY-ZYuanHYeY-QZengS. Preparation and characterization of stearic acid nanostructured lipid carriers by solvent diffusion method in an aqueous system. Colloids Surf B: Biointerfaces (2005) 45:167–73. 10.1016/j.colsurfb.2005.08.005
43.
SarmentoBMazzagliaDBonferoniMCNetoAPdo Céu MonteiroMSeabraV. Effect of chitosan coating in overcoming the phagocytosis of insulin loaded solid lipid nanoparticles by mononuclear phagocyte system. Carbohydr Polymers (2011) 84:919–25. 10.1016/j.carbpol.2010.12.042
44.
AhmaditabarPMomtazi‐BorojeniAARezayanAHMahmoodiMSahebkarAMellatM. Enhanced entrapment and improved in vitro controlled release of n‐acetyl cysteine in hybrid PLGA/lecithin nanoparticles prepared using a nanoprecipitation/self‐assembly method. J Cellular Biochemistry (2017) 118:4203–9. 10.1002/jcb.26070
45.
PanXMLiJGanRHuXN. Preparation and in vitro evaluation of enteric-coated tablets of rosiglitazone sodium. Saudi Pharm J (2015) 23:581–6. 10.1016/j.jsps.2015.02.018
46.
AnalAKStevensWF. Chitosan–alginate multilayer beads for controlled release of ampicillin. Int Journal Pharmaceutics (2005) 290:45–54. 10.1016/j.ijpharm.2004.11.015
47.
ChandrasekaranARJiaCYThengCSMuniandyTMuralidharanSDhanarajSA. Invitro studies and evaluation of metformin marketed tablets-Malaysia. J Applied Pharmaceutical Science (2011) 214–7.
48.
KhalilNMdo NascimentoTCFCasaDMDalmolinLFde MattosACHossIet alPharmacokinetics of curcumin-loaded PLGA and PLGA–PEG blend nanoparticles after oral administration in rats. Colloids Surf B: Biointerfaces (2013) 101:353–60. 10.1016/j.colsurfb.2012.06.024
49.
SunBYanHLiCYinLLiFZhouLet alBeneficial effects of walnut (Juglans regia L.) oil-derived polyunsaturated fatty acid prevents a prooxidant status and hyperlipidemia in pregnant rats with diabetes. Nutr and Metabolism (2020) 17:1–11. 10.1186/s12986-020-00514-3
50.
VeerapurVPratapVThippeswamyBMariettaPBansalPKulkarniPet alPolyphenolic enriched extract of Cassia glauca Lamk, improves streptozotocin-induced type-1 diabetes linked with partial insulin resistance in rats. J Ethnopharmacology (2017) 198:489–98. 10.1016/j.jep.2017.01.025
51.
WangWZhaGZouJ-jWangXLiC-nWuX-j. Berberine attenuates cigarette smoke extract-induced airway inflammation in mice: involvement of TGF-β1/Smads signaling pathway. Curr Medical Science (2019) 39:748–53. 10.1007/s11596-019-2101-8
52.
ZhouYLiuS-qPengHYuLHeBZhaoQ. In vivo anti-apoptosis activity of novel berberine-loaded chitosan nanoparticles effectively ameliorates osteoarthritis. Int Immunopharmacology (2015) 28:34–43. 10.1016/j.intimp.2015.05.014
53.
WuS-JDonT-MLinC-WMiF-L. Delivery of berberine using chitosan/fucoidan-taurine conjugate nanoparticles for treatment of defective intestinal epithelial tight junction barrier. Mar Drugs (2014) 12:5677–97. 10.3390/md12115677
54.
XueMZhangLYangM-xZhangWLiX-mOuZ-met alBerberine-loaded solid lipid nanoparticles are concentrated in the liver and ameliorate hepatosteatosis in db/db mice. Int Journal Nanomedicine (2015) 10:5049–57. 10.2147/ijn.s84565
55.
XueMYangM-xZhangWLiX-mGaoD-hOuZ-met alCharacterization, pharmacokinetics, and hypoglycemic effect of berberine loaded solid lipid nanoparticles. Int J Nanomedicine (2013) 8:4677. 10.2147/ijn.s51262
56.
CloseBBanisterKBaumansVBernothE-MBromageNBunyanJet alRecommendations for euthanasia of experimental animals: part 2. Lab Animals (1997) 31:1–32. 10.1258/002367797780600297
57.
CloseBBanisterKBaumansVBernothE-MBromageNBunyanJet alRecommendations for euthanasia of experimental animals: part 1. Lab Animals (1996) 30:293–316. 10.1258/002367796780739871
58.
SiahaanJMIllyasSLindartoDNainggolanM. The effect of ethanol and ethyl acetate fraction of chayote fruit (Sechium edule jacq. swartz) on the oxidative stress and insulin resistance of male white rat model type 2 diabetes mellitus. Open Access Macedonian J Med Sci (2020) 8:962–9. 10.3889/oamjms.2020.4517
59.
IbrahimMAHabilaJDKoorbanallyNAIslamMS. Butanol fraction of Parkia biglobosa (Jacq.) G. Don leaves enhance pancreatic β-cell functions, stimulates insulin secretion and ameliorates other type 2 diabetes-associated complications in rats. J Ethnopharmacology (2016) 183:103–11. 10.1016/j.jep.2016.02.018
60.
KunasegaranTMustafaMRMuruganDDAchikeFI. The bioflavonoid quercetin synergises with PPAR-γ agonist pioglitazone in reducing angiotensin-II contractile effect in fructose-streptozotocin induced diabetic rats. Biochimie (2016) 125:131–9. 10.1016/j.biochi.2016.03.008
61.
LuoYZhangYPanKCritzerFDavidsonPMZhongQ. Self-emulsification of alkaline-dissolved clove bud oil by whey protein, gum arabic, lecithin, and their combinations. J Agricultural Food Chemistry (2014) 62:4417–24. 10.1021/jf500698k
62.
GanesanPRamalingamPKarthivashanGKoYTChoiD-K. Recent developments in solid lipid nanoparticle and surface-modified solid lipid nanoparticle delivery systems for oral delivery of phyto-bioactive compounds in various chronic diseases. Int Journal Nanomedicine (2018) 13:1569–83. 10.2147/ijn.s155593
63.
ShiriSGharanjigKHeidariHTahghighiA. Plant sources containing berberine with potential of control type 2 diabetes mellitus: a brief literature review. Funct Food Sci (2024) 4:466–78. 10.31989/ffs.v4i12.1461
64.
AskariVRKhosraviKBaradaran RahimiVGarzoliS. A mechanistic review on how berberine use combats diabetes and related complications: molecular, cellular, and metabolic effects. Pharmaceuticals (2023) 17(7):7. 10.3390/ph17010007
65.
Araj-KhodaeiMAyatiMHAzizi ZeinalhajlouANovinbahadorTYousefiMShiriMet alBerberine-induced glucagon-like peptide-1 and its mechanism for controlling type 2 diabetes mellitus: a comprehensive pathway review. Arch Physiol Biochem (2024) 130:678–85. 10.1080/13813455.2023.2258559
66.
ShrivastavaSSharmaASaxenaNBhamraRKumarS. Addressing the preventive and therapeutic perspective of berberine against diabetes. Heliyon (2023) 9:e21233. 10.1016/j.heliyon.2023.e21233
67.
MiaoRZhangBZhouDKangMTianJZhaoLet alClinical efficacy of curcumin, resveratrol, silymarin, and berberine on Cardio‐Metabolic risk factors among patients with type 2 diabetes mellitus: a systemic review and bayesian network meta‐analysis. Phytotherapy Research: PTR (2025). 10.1002/ptr.8431
68.
GuoJChenHZhangXLouWZhangPQiuYet alThe effect of berberine on metabolic profiles in type 2 diabetic patients: a systematic review and meta‐analysis of randomized controlled trials. Oxidative Med Cell Longevity (2021) 2021:2074610. 10.1155/2021/2074610
69.
WangJBiCXiHWeiF. Effects of administering berberine alone or in combination on type 2 diabetes mellitus: a systematic review and meta-analysis. Front Pharmacol (2024) 15:1455534. 10.3389/fphar.2024.1455534
70.
MansourASajjadi-JaziSMGeramiHKhorasanianASMoalemzadehBKarimiSet alThe efficacy and safety of berberine in combination with cinnamon supplementation in patients with type 2 diabetes: a randomized clinical trial. Eur J Nutr (2025) 64:102. 10.1007/s00394-025-03618-9
71.
JiLMaJMaYChengZGanSYuanGet alBerberine ursodeoxycholate for the treatment of type 2 diabetes: a randomized clinical trial. JAMA Netw Open (2025) 8:e2462185–e85. 10.1001/jamanetworkopen.2024.62185
72.
ChaudhariSDalabeheraMSubudhiRNDuaKKaurMPaudelKRet alFrom nature to nanotech: unlocking berberine’s therapeutic approaches. J Drug Deliv Sci Technology (2025) 108:106924. 10.1016/j.jddst.2025.106924
73.
KhanMJHafeezASiddiquiMA. Nanocarrier based delivery of berberine: a critical review on pharmaceutical and preclinical characteristics of the bioactive. Curr Pharm Biotechnol (2023) 24:1449–64. 10.2174/1389201024666230112141330
74.
WuJYanLJ. Streptozotocin-induced type 1 diabetes in rodents as a model for studying mitochondrial mechanisms of diabetic β cell glucotoxicity. Diabetes Metab Syndr Obes Targets Ther (2015) 8:181. 10.2147/dmso.s82272
75.
KhalafYHDawoodYKhashanAA. Green biosynthesis of berberine-mediated silver nanorods: their protective and antidiabetic effects in streptozotocin-induced diabetic rats. Results Chem (2023) 5:100722. 10.1016/j.rechem.2022.100722
76.
WangZWuJZhouQWangYChenT. Berberine nanosuspension enhances hypoglycemic efficacy on streptozotocin induced diabetic C57BL/6 mice. Evidence‐Based Complement Altern Med (2015) 2015:239749. 10.1155/2015/239749
77.
ZhouJZhouSTangJZhangKGuangLHuangYet alProtective effect of berberine on beta cells in streptozotocin-and high-carbohydrate/high-fat diet-induced diabetic rats. Eur J Pharmacol (2009) 606:262–8. 10.1016/j.ejphar.2008.12.056
78.
LengS-hLuF-EXuL-j. Therapeutic effects of berberine in impaired glucose tolerance rats and its influence on insulin secretion. Acta Pharmacologica Sinica (2004) 25:496–502.
79.
WangZ-QLuF-ELengS-HFangX-SChenGWangZ-Set alFacilitating effects of berberine on rat pancreatic islets through modulating hepatic nuclear factor 4 alpha expression and glucokinase activity. World J Gastroenterol (2008) 14:6004. 10.3748/wjg.14.6004
80.
GaoNZhaoTYLiXJ. The protective effect of berberine on β-cell lipoapoptosis. J Endocrinological Invest (2011) 34:124–30. 10.1007/bf03347042
81.
LiMShenYWangQZhouX. MiR-204-5p promotes apoptosis and inhibits migration of osteosarcoma via targeting EBF2. Biochimie (2019) 158:224–32. 10.1016/j.biochi.2018.12.003
82.
ChangWChenLHatchGM. Berberine as a therapy for type 2 diabetes and its complications: from mechanism of action to clinical studies. Biochem Cell Biol (2015) 93:479–86. 10.1139/bcb-2014-0107
83.
LuS-SYuY-LZhuH-JLiuX-DLiuLLiuY-Wet alBerberine promotes glucagon-like peptide-1 (7-36) amide secretion in streptozotocin-induced diabetic rats. J Endocrinol (2009) 200:159–65. 10.1677/joe-08-0419
84.
TengholmAGylfeE. cAMP signalling in insulin and glucagon secretion. Diabetes Obesity Metabolism (2017) 19:42–53. 10.1111/dom.12993
85.
YuYLiuLWangXLiuXLiuXXieLet alModulation of glucagon-like peptide-1 release by berberine: in vivo and in vitro studies. Biochem Pharmacology (2010) 79:1000–6. 10.1016/j.bcp.2009.11.017
86.
HardieDG. AMP-activated protein kinase: a master switch in glucose and lipid metabolism. Rev Endocr Metab Disord (2004) 5:119–25. 10.1023/b:remd.0000021433.63915.bb
87.
KahnBBAlquierTCarlingDHardieDG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metabolism (2005) 1:15–25. 10.1016/j.cmet.2004.12.003
88.
BrusqJ-MAncellinNGrondinPGuillardRMartinSSaintillanYet alInhibition of lipid synthesis through activation of AMP kinase: an additional mechanism for the hypolipidemic effects of berberine. J Lipid Res (2006) 47:1281–8. 10.1194/jlr.m600020-jlr200
89.
KimSHShinE-JKimE-DBayaraaTFrostSCHyunC-K. Berberine activates GLUT1-mediated glucose uptake in 3T3-L1 adipocytes. Biol Pharm Bull (2007) 30:2120–5. 10.1248/bpb.30.2120
90.
LeeYSKimWSKimKHYoonMJChoHJShenYet alBerberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes (2006) 55:2256–64. 10.2337/db06-0006
91.
ChengZPangTGuMGaoA-HXieC-MLiJ-Yet alBerberine-stimulated glucose uptake in L6 myotubes involves both AMPK and p38 MAPK. Biochim Biophys Acta (BBA)-General Subjects (2006) 1760:1682–9. 10.1016/j.bbagen.2006.09.007
92.
YinJGaoZLiuDLiuZYeJ. Berberine improves glucose metabolism through induction of glycolysis. Am J Physiology-Endocrinology Metab (2008) 294:E148–E156. 10.1152/ajpendo.00211.2007
93.
GiuglianoDCerielloAPaolissoG. Oxidative stress and diabetic vascular complications. Diabetes CARE (1996) 19:257–67. 10.2337/diacare.19.3.257
94.
WidowatiW. Potensi antioksidan sebagai antidiabetes. Jkm (2008) 7:1–11.
95.
BajajSKhanA. Antioxidants and diabetes. Indian Journal Endocrinol And Metab (2012) 16:S267–S71. 10.4103/2230-8210.104057
96.
CerielloATestaR. Antioxidant anti-inflammatory treatment in type 2 diabetes. Diabetes Care (2009) 32:S232–S236. 10.2337/dc09-s316
97.
LockmanPROyewumiMOKoziaraJMRoderKEMumperRJAllenDD. Brain uptake of thiamine-coated nanoparticles. J Controlled Release (2003) 93:271–82. 10.1016/j.jconrel.2003.08.006
98.
AlbaneseATangPSChanWC. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu Rev Biomed Engineering (2012) 14:1–16. 10.1146/annurev-bioeng-071811-150124
99.
GrattonSERoppPAPohlhausPDLuftJCMaddenVJNapierMEet alThe effect of particle design on cellular internalization pathways. Proc Natl Acad Sci (2008) 105:11613–18. 10.1073/pnas.0801763105
Summary
Keywords
berberine, chitosan, gestational diabetes mellitus, rat, solid lipid nanoparticle
Citation
Liu Y, Hussain SA and Yue H (2025) Protective effects of berberine-loaded chitosan/solid lipid nanoparticles in streptozotocin-induced gestational diabetes mellitus rats. Exp. Biol. Med. 250:10749. doi: 10.3389/ebm.2025.10749
Received
14 July 2025
Revised
08 October 2025
Accepted
20 November 2025
Published
12 December 2025
Volume
250 - 2025
Updates
Copyright
© 2025 Liu, Hussain and Yue.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hua Yue, yuehua10288@outlook.com, yuehua1028@sina.com
ORCID: Hua Yue, orcid.org/0009-0002-9357-892X
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.