Abstract
Attention deficit/hyperactivity disorder is a common neuropsychiatric disorder that affects around 5%–7% of children worldwide. Artificial intelligence provides advanced models and algorithms for better diagnosis, prediction and classification of attention deficit/hyperactivity disorder. This study aims to explore artificial intelligence models used for the prediction, early diagnosis and classification of attention deficit/hyperactivity disorder as reported in the literature. A scoping review was conducted and reported in line with the PRISMA-ScR (Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews) guidelines. Out of the 1994 publications, 52 studies were included in the scoping review. The included articles reported the use of artificial intelligence for 3 different purposes. Of these included articles, artificial intelligence techniques were mostly used for the diagnosis of attention deficit/hyperactivity disorder (38/52, 79%). Magnetic resonance imaging (20/52, 38%) were the most frequently used data in the included articles. Most of the included articles used data sets with a size of <1,000 samples (28/52, 54%). Machine learning models were the most prominent branch of artificial intelligence used for attention deficit/hyperactivity disorder in the studies, and the support vector machine was the most used algorithm (34/52, 65%). The most commonly used validation in the studies was k-fold cross-validation (34/52, 65%). A higher level of accuracy (98.23%) was found in studies that used Convolutional Neural Networks algorithm. This review provides an overview of research on artificial intelligence models and algorithms for attention deficit/hyperactivity disorder, providing data for further research to support clinical decision-making in healthcare.
Impact statement
At present, artificial intelligence is a hot topic, but it still needs to be developed in the medical field, especially in pediatric clinical research. We believe that the researchability of artificial intelligence is sufficient. As we know, in the medical field, early diagnosis and identification of a certain clinical disease is crucial for clinical doctors, and the emergence of artificial intelligence is likely to bring tremendous assistance to clinical diagnosis and treatment work. In this study, we conducted scope evaluation according to the PRISMA-ScR guidelines, and mainly summarized AI models and algorithms for diagnosis, prediction, and classification of attention deficit/hyperactivity disorder. The hope is to provide clinical decisions that support future research in healthcare.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a neurodevelopmental disorder caused by the interaction of genetic and environmental factors that has a worldwide prevalence of 7.2% in children [1, 2]. ADHD is characterized by a persistent and impairing pattern of inattention and/or hyperactivity/impulsivity, about 60% of children with ADHD have symptoms that persist into adulthood [3], and 89% of ADHD patients are accompanied by mental illness, representing a significant public health problem [4]. Therefore, early diagnosis of ADHD is critical to enable early intervention and treatment.
At present, the diagnosis of ADHD mainly relies on the judgment of psychiatrists, based primarily on reports from parents and teachers, behavioral observations, and clinical interviews, which are sensitive to subjective biases [5, 6]. Existing studies have shown that ADHD is a highly heterogeneous disease involving multiple etiological and risk factors, with different clinical characteristics, development process and outcome, which brings diagnostic challenges to clinicians, and false positive diagnosis or misdiagnosis may occur in clinical practice [7, 8]. It has been shown that a significant association between disease and trait does not necessarily imply that it can be used for disease prediction. Neuroimaging plays a vital role in the study of brain function by visualizing the structure and activity of the brain, allowing researchers to understand how different brain regions are involved in various cognitive and behavioral processes [9]. The brains of children with ADHD are different in terms of structure and function, and these differences are also associated with neurocognitive performance. Structural magnetic resonance imaging (sMRI), functional MRI (fMRI), resting-state fMRI (rs-fMRI) and diffusion tensor imaging (DTI) were used to characterize the etiology and phenotype of ADHD from different dimensions [10]. Genome-wide association studies have also revealed several variants in ADHD [11, 12]. In addition, other studies have attempted to use electrocardiogram (ECG) signals [13], eye tracking [14], physiological signals, wearable device data [15], and exercise data to help diagnose ADHD.
Artificial intelligence (AI) is a technology with great potential in medicine, machine learning (ML) is a powerful tool for making critical decisions by analyzing large data sets such as social behavior patterns and various health conditions, deep learning (DL) is a branch of ML [16]. Many neurological diseases are identified based on subjective diagnostic criteria. Neuroimaging is a promising objective diagnostic tool. The task of ML is to model the relationship between features extracted from imaging data and individual labels in the data set, which can be used for new or invisible data sets. It creates broad prospects for disease diagnosis, prognosis and management in health care and enriches personalized medicine [17]. With the increasing popularity of AI models, AI technology has achieved satisfactory results in the diagnosis of brain-related diseases such as Alzheimer’s disease, Parkinson’s disease, autism spectrum disorder (ASD) [18], and ADHD is no exception. AI can assist in ADHD diagnosis, classification, prognosis, treatment prediction, and the development of new therapeutic drugs.
A large number of articles have been published on AI technologies for ADHD. Several reviews were conducted to summarize previous studies; however, they had the following limitations: First, they focused on studies of ADHD diagnosis with machine learning methods using MRI data [19]; Second, they focused on describing the efficacy of ML or DL models in the diagnosis, classification, or prediction of ADHD, without describing in detail the characteristics of the AI algorithms used [20]. The available literature lacks a review that provides an overview of the features of the AI algorithms used in ADHD. Thus, this review aims to explore the characteristics of AI models used for the diagnosis, prediction and classification to aid scientists advance research on this field.
Materials and methods
Overview
In this scoping review, we conducted a systematic literature search that reviewed research involving the use of AI for ADHD prediction, classification, and diagnosis. To ensure the transparency and reliability of this study, the literature search was conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols Extension for Scoping Reviews (PRISMA-ScR) guidelines [21]. The protocols used in the scoping review are detailed in the following sections.
Search strategy
Search sources
Two authors (Bo Sun and Fei Cai) conducted an independent search in February 2025 and screened abstracts and full texts, which were finally checked by the corresponding author (Bing Wei). During this period, we searched four online databases, including MedRXiv, BioRXiv, PubMed, and Science Direct. The search focused on both medical and computer science databases.
Search terms
We used the following items as keywords: (“artificial intelligence” OR “machine learning” OR “deep learning” OR “supervised learning” OR “unsupervised learning” OR “reinforcement learning”) AND (“attention-deficit/hyperactivity disorder”) AND (diagnosis* OR detect* OR predict* OR screen*). For more information on the exact search terms used to search each database, see Multimedia Supplementary Appendix S1.
Eligibility criteria
The studies included in this review mainly concerned AI technologies for ADHD diagnosis and risk prediction. In other words, we focus on AI models related to ADHD diagnosis. The search was limited to original journal research articles in English. We excluded articles (i.e., literature reviews, dissertations) outlining AI approaches to ADHD as well as studies based purely on clinical trials and experimental studies. Inclusion criteria include: (1) AI technology; (2) the goal to diagnose or screen for ADHD; (3) participants are children only; (4) the data is publicly available. Exclusion criteria include: (1) inadequate details in terms of AI models; (2) same raw data; (3) inappropriate article types (e.g., case reports, reviews, papers, proposals, conference abstracts, editorials, generic manuscripts, and reviews).
Study selection
Articles selected from each database were charted on Microsoft Excel. At the same time, we imported all the retrieved articles into the EndNote software, and the duplicate check function was used to remove duplicate studies. Titles and abstracts were carefully selected and screened, and articles were searched for full text reading if they met the inclusion criteria. Any disagreements were resolved through discussion among the investigators. To measure agreement between investigators, we calculated the Cohen kappa [22], where the screening result for title and abstract was 0.976, while the screening result for full text was 0.82. We documented the inter-investigator agreement matrix in Multimedia Supplementary Appendix S2.
Data extraction
The investigators performed the data extraction process using a pre-designed standardized form (Multimedia Supplementary Appendix S3). The extracted data included: (1) author, country, and year; (2) the age, number and health status of the participants; (3) the source, setting, and availability of the data used by AI; (4) algorithms, types, and features of AI models; (5) outcomes of AI diagnosis of ADHD.
Results
Search results
We preliminarily identified 1994 articles using four open online databases: PubMed (n = 613), Science Direct (n = 666), BioRXiv (n = 542), and MedRxiv (n = 173). After that, we excluded 557 duplicate articles. Of the remaining studies, 1,195 articles were removed after title and abstract screening. In addition, 13 articles were not searchable, so 229 articles were included in the full-text screening. As shown in Figure 1, after reviewing the full text, we excluded 177 articles for a variety of reasons. A total of 52 articles met our inclusion criteria and were included in this scoping review.
FIGURE 1
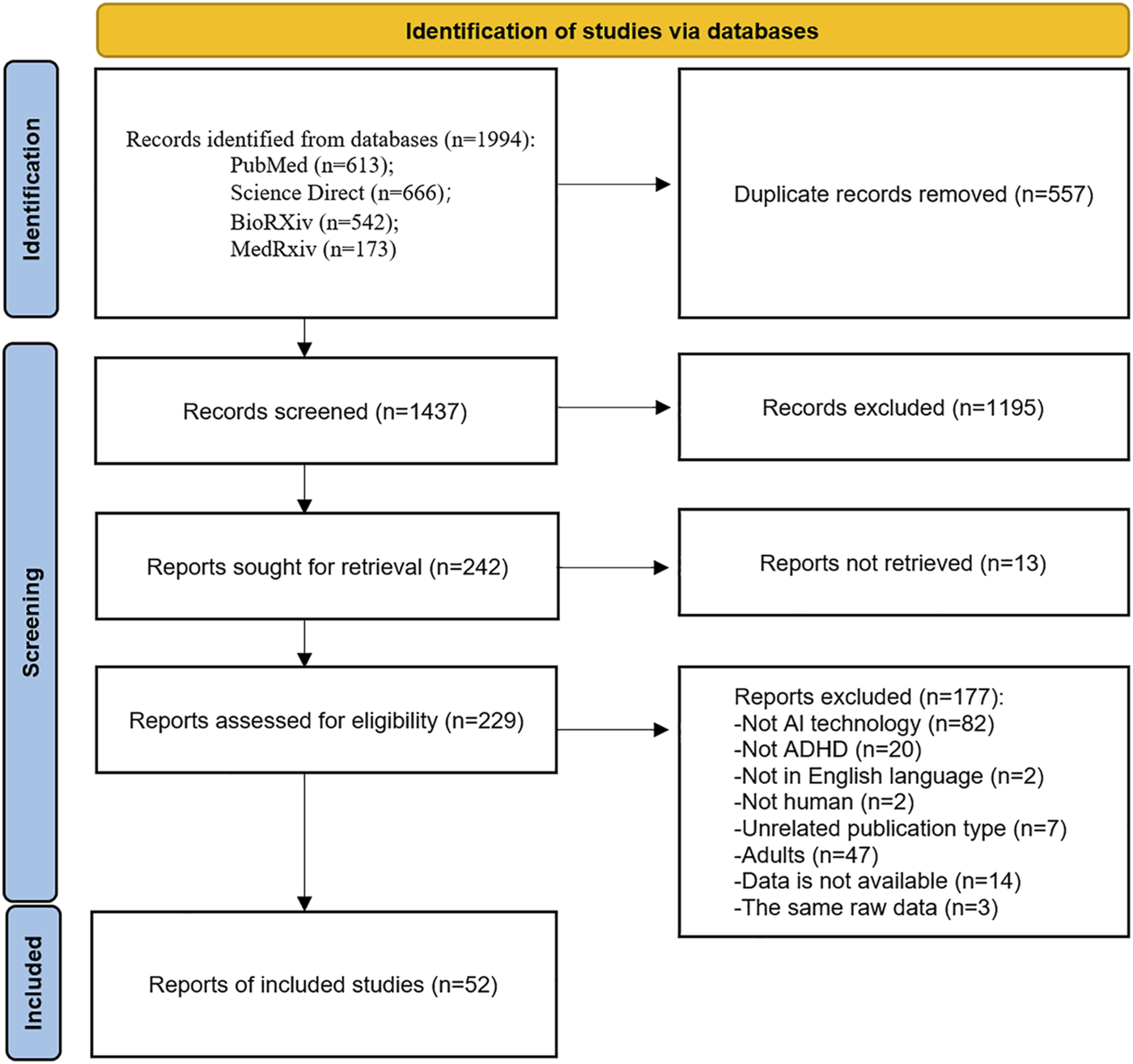
PRISMA-ScR flowchart of the study selection.
Main characteristics of the included articles
Characteristics of the included studies were shown in Table 1. All of the studies we included were published in peer-reviewed journals (52/52, 100%). Eligible studies were published between 2012 and 2025, mainly in China (16/52, 31%), followed by Korea (9/52, 17%). The number of participants mentioned in the included studies ranged from 10 to 238,696. Of these, 33 studies reported the proportion of female participants, ranging from 2% to 50%. Furthermore, 88% (46/52) of the included studies only recruited participants with ADHD, and 12% (6/52) of the studies included participants with other medical conditions. Multimedia Supplementary Appendix S4 showed the detailed characteristics of the included studies.
TABLE 1
| Characteristics | Studies n (%) | References |
|---|---|---|
| Publication type | ||
| Journal articles | 52 (100) | [12, 13, 15, 23–28], [29–71] |
| Year of publication, n (%) | ||
| 2025 | 1 (1.9) | [26] |
| 2024 | 9 (17.3) | [26, 63, 64, 66–71] |
| 2023 | 8 (15.4) | [35, 42, 47, 50] |
| 2022 | 7 (13.5) | [23, 27, 33, 39, 45, 60, 61] |
| 2021 | 4 (7.7) | [12, 13, 24, 38] |
| 2020 | 4 (7.7) | [25, 37, 44, 52] |
| 2012–2019 | 19 (36.5) | [28–32, 34, 36, 40–44, 48, 54–56, 58, 59, 62] |
| Country of publication | ||
| China | 16 (31) | [12, 24, 28, 29, 39, 46, 54, 59, 61, 64, 66, 68–71] |
| Korea | 9 (17) | [15, 25, 30, 47, 49, 50, 55, 58] |
| United States | 7 (13) | [23, 32, 38, 40, 41, 52, 57] |
| Canada | 2 (4) | [31, 43] |
| Germany | 2 (4) | [34, 60] |
| Spain | 3 (6) | [48, 56, 63] |
| Australia | 1 (2) | [53] |
| Denmark | 1 (2) | [67] |
| Iran | 1 (2) | [33] |
| Israel | 1 (2) | [37] |
| India | 1 (2) | [42] |
| Italy | 1 (2) | [62] |
| Japan | 1 (2) | [44] |
| Minnesota | 1 (2) | [36] |
| Singapore | 1 (2) | [13] |
| Sweden | 1 (2) | [27] |
| Turken | 1 (2) | [35] |
| Türkiye | 1 (2) | [51] |
| United Kingdom | 1 (2) | [45] |
| Number of participants, n (%) | ||
| <99 | 17 (33) | [25, 30, 34–36, 38, 47–49, 52, 56, 58, 62, 66, 69–71] |
| 100–999 | 28 (54) | [13, 15, 23, 26, 28, 29, 31, 33, 37, 39–44, 46, 50, 51, 54, 55, 59–61, 63–65, 67, 68] |
| >1,000 | 7 (13) | [12, 24, 27, 32, 45, 53, 57] |
| Gender, range (%) | ||
| Female | 2–50 | [12, 13, 15, 24, 28, 30, 31, 33, 35–40, 42, 44–47, 52, 55–60, 62–65, 67, 69, 70] |
| Participants’ health conditions, n (%) | ||
| Only ADHD | 46 (88) | [12, 13, 15, 23–31, 34–39], [41–52, 54–60, 62–66, 68–71] |
| ADHD and OTHERS | 6 (12) | [32, 33, 40, 53, 61, 67] |
Characteristics of the included studies (n = 52).
Characteristics of AI techniques for ADHD
Of the included studies, 76.9% used only ML algorithms, 9.6% used only DL algorithms, and 13.5% applied ML including DL algorithms. In addition, we collated the AI models, algorithms, and methods used in the included ADHD studies. The most commonly used model was support vector machine (SVM, 34/52, 65%), followed by random forest (RF, 17/52,33%). In the 52 studies, AI algorithms were used for 3 different purposes. The most common purposes were early diagnosis (38/52,79%) and risk predictions (10/52, 14%; Table 2). Only 11 studies stated the programming languages used to develop the models, and they were R (5/52, 10%) and Python (6/52,12%). Multimedia Supplementary Appendix S5 showed the characteristics of the AI techniques used in each study.
TABLE 2
| Types | Studies n (%) | References |
|---|---|---|
| AI type | ||
| ML | 40 (76.9) | [13, 15, 23–26, 28–34, 36, 38, 39, 41–49, 52–56, 58, 60, 62–65, 67–70] |
| DL | 5 (9.6) | [35, 50, 51, 61, 66] |
| ML and DL | 7 (13.5) | [12, 27, 37, 40, 57, 61, 71] |
| AI algorithms/models/methods a | ||
| SVM | 34 (65) | [12, 13, 23, 24, 28–34, 39–46, 48, 49, 52, 54–60, 62, 63, 67, 68, 70] |
| RF | 17 (33) | [12, 15, 24, 27, 32, 33, 36–38, 40, 42, 45, 53, 63, 69–71] |
| DT | 10 (19) | [13, 31, 32, 38, 52, 53, 63, 68, 69, 71] |
| Gradient boosting | 7 (13) | [15, 26, 27, 33, 63, 69, 70] |
| K-nearest neighbors (KNN) | 7 (13) | [27, 31, 38, 42, 43, 45, 68–70] |
| AdaBoost | 6 (12) | [13, 27, 36, 63, 69, 70] |
| LR | 6 (12) | [27, 32, 38, 42, 43, 45] |
| Convolutional neural network (CNN) | 5 (10) | [12, 35, 51, 56, 59, 61] |
| Naive bayes (NB) | 5 (10) | [12, 35, 51, 59, 61, 63] |
| Extreme learning machine (ELM) | 3 (6) | [30, 54, 55] |
| Multi-layer perceptron (MLP) | 3 (6) | [45, 59, 63] |
| Neural network (NN) | 3 (6) | [37, 40, 63] |
| Deep-learning neural network (DNN) | 2 (4) | [27, 66] |
| Linear discriminant (LDA) | 2 (4) | [32, 47] |
| Multinomial regression (MR) | 2 (4) | [40] |
| Recurrent neural network (RNN) | 2 (4) | [50, 71] |
| Categorical lasso | 1 (2) | [32] |
| Classification and regression tree (CART) | 1 (2) | [70] |
| Elastic net regularization (EN) | 1 (2) | [58] |
| Partial least squares (PLS) | 1 (2) | [40] |
| Purpose of AI algorithms | ||
| Early diagnosis | 38 (79) | [15, 24, 25, 28–43, 47, 49–57, 59–62, 65–67, 70, 71] |
| Predicting | 10 (14) | [12, 23, 26, 27, 44, 45, 58, 63, 64, 68] |
| Classification | 4 (7) | [13, 46, 48, 69] |
| Programming languages b | ||
| Python | 6 (12) | [23, 26, 27, 32, 53, 64] |
| R | 5 (10) | [25, 39, 41, 44, 47] |
Types of AI techniques used for ADHD (n = 52 studies).
Some studies used more than one model.
Only 9 studies reported the programming languages used to develop the model.
Table 3 showed the different data categories used in the included studies: 38% of the studies (20/52) involved brain imaging, 25% (13/52) included demographic information, 19% (10/52) used electroencephalogram (EEG), and so on. 60% of the included studies used datasets from closed-source (i.e., data collected directly from databases of study participants or clinical settings) and 40% from open-source (i.e., publicly available databases). The numbers of features used to develop the models in the included studies ranged from 3 to 13,585,634. And 25 studies (48%) did not exceed 100 features in developing their model. We provided a detailed description of the number of features and data categories of the included studies in Multimedia Supplementary Appendix S6.
TABLE 3
| Features | Studies n (%) | References |
|---|---|---|
| Data category a | ||
| Brain imaging | 20 (38) | [23–25, 29–31, 33, 35, 37, 41–43, 46, 54–58, 65, 67] |
| Demographic information | 13 (25) | [28, 29, 37, 38, 41–45, 58, 60, 67] |
| EEG measurements | 10 (19) | [13, 26, 34, 39, 49, 51, 59, 61, 66, 71] |
| Parent/Teacher report questionnaire | 9 (17) | [32, 36, 45, 52, 53, 60, 63, 68, 71] |
| Neurocognitive features | 7 (13) | [36, 37, 44, 50, 52, 60, 62] |
| Eye tracking | 3 (6) | [48, 64, 66] |
| Genetic characteristics | 3 (6) | [12, 25, 58] |
| Behavioral data | 2 (4) | [69, 70] |
| Wearable data | 2 (4) | [15, 40] |
| Others | 3 (6) | [28, 47, 62] |
| Number of features | ||
| <99 | 25 (48) | [13, 15, 26, 27, 32–34, 36, 40, 43, 45, 46, 52, 58–60, 62–65, 67–71] |
| 100–999 | 10 (19) | [23, 29, 30, 38, 42, 47, 54, 56, 57, 66] |
| >1,000 | 9 (15) | [12, 24, 25, 31, 41, 49, 51, 53, 55] |
| Not reported | 8 (15) | [28, 35, 37, 39, 44, 48, 50] |
| Type of data set source | ||
| Closed | 31 (60) | [12, 13, 15, 23, 26, 34–40, 44, 49, 50, 52, 56–60, 62–71] |
| Open | 21 (40) | [24, 25, 27–33, 40–42, 44, 46–48, 51, 53–55, 61] |
Features and categories of data used in the included articles (n = 52 studies).
Many studies used more than one data category.
As shown in Table 4, the included studies used different validation techniques in the development of AI models, mainly of two. Among them, k-fold CV (34/52, 65%) is the more commonly used method. Only 13% of studies (7/52) mentioned confusion matrices, but all 52 studies mentioned performance metrics for AI models. According to statistics, the most commonly used performance measure was accuracy (ACC, 45/52, 87%). In Table 5, 8 studies reported the precision of AI algorithms, ranging from 80% to 95%, with an average of 92.53%; The area under the curve (AUC) in 26 studies ranged from 57.6% to 99.64%, with a mean of 83.77%; The mean ACC of the 45 studies was 83.06%, ranging from 53.2% to 98.23%; 35 studies reported specificities varying between 58.8% and 99.11%, with a mean of 84.08%; The F1-score valued in 11 studies ranged from 48.89% to 95%, with a mean of 85.21%. In addition, the sensitivity of the AI algorithms reported in 35 studies ranged from 33% to 98.24%, with an average of 74.67%.
TABLE 4
| Validation and statistics | Studies n (%) | References |
|---|---|---|
| Validation approach a | ||
| K-fold CV | 34 (65) | [13, 24, 27–35, 37–39, 41–43, 45, 46, 48, 51, 53, 57–64, 68–71] |
| LOOCV | 10 (19) | [25, 26, 28, 47, 50, 52, 54, 56, 61, 62] |
| Not reported | 11 (21) | [12, 15, 23, 36, 40, 44, 49, 55, 65–67] |
| Confusion matrix | ||
| Reported | 7 (13) | [35, 36, 50, 51, 53, 61, 63] |
| Not reported | 45 (87) | [12, 13, 15, 23–34], [37–49, 52, 54–60, 62, 64–71] |
| Performance measures b | ||
| ACC | 45 (87) | [12, 13, 23–31, 33–43], [46–50, 52–54, 56–64, 66–71] |
| Sensitivity | 35 (67) | [12, 13, 15, 24, 26–29, 31, 33, 36–42, 44, 46, 47, 50–53, 55–57, 60–64, 67, 69, 71] |
| Specificity | 35 (67) | [12, 13, 15, 24, 26–29, 31, 33, 36–42, 44, 46, 47, 50–53, 55–57, 60–64, 67, 69, 70] |
| AUC | 26 (50) | [12, 15, 24, 25, 27, 29, 32, 35, 38, 40, 45, 46, 48, 49, 53, 54, 58–65, 69, 70] |
| F1-score | 11 (21) | [23, 26, 35, 45, 46, 50, 55, 61, 64, 68, 71] |
| Precision | 8 (15) | [26, 35, 45, 53, 55, 64, 68, 71] |
| Recall | 5 (10) | [35, 45, 55, 69, 71] |
| False-negative | 3 (6) | [36, 48, 50] |
| False-positive | 3 (6) | [36, 48, 50] |
| Negative predictive value | 3 (6) | [15, 27, 33] |
| Positive predictive value | 2 (4) | [15, 33] |
| Kappa | 1 (2) | [40] |
| J-statistic | 1 (2) | [46] |
| Positive predicted power | 1 (2) | [27] |
| True-negative | 1 (2.4) | [36] |
| True-positive | 1 (2.4) | [36] |
Validation approaches and performance measures (n = 52 studies).
Total number does not add up, as many studies used more than one validation method.
Total number does not add up, as many studies used more than one performance measure.
TABLE 5
| Performance measures | Results (%), mean (range) |
|---|---|
| Precision | 92.53 (80–95) |
| AUC | 83.77 (57.6–99.64) |
| ACC | 83.06 (53.2–98.23) |
| Specificity | 84.08 (58.8–99.11) |
| F1-score | 85.21 (48.89–95) |
| Sensitivity | 74.67 (33–98.24) |
Overview of performance of AI models.
Discussion
Principal findings
In this study, we explored the application of AI techniques in the early diagnosis, prediction, and classification of ADHD. We searched articles published from January 2012 to February 2025, and of the 1994 articles retrieved, 52 were eventually included in our scoping review. Over the past 4 years, an increasing number of studies have been published: 9 in 2024, 8 in 2023, 7 in 2022, and 4 in 2021. Tracing its causes, the digital innovation process has stimulated the increasing demand for telemedicine programs, and healthcare systems have increasingly relied on AI technology [72]. In the field of child and adolescent neuropsychiatry, the development and use of online platforms for collecting case histories, demographic, and behavioral information have been steadily increasing [73]. The increase in available data has provided new opportunities for cutting-edge methods such as ML and DL, which used high-dimensional data to build predictive models to capture non-linear relationships across multiple data sources, traditional statistical methods could not achieve [74]. The articles we included focused on AI being used for three purposes in ADHD: early diagnosis, classification, and prediction. None of the included articles were used for other purposes, such as treatment response prediction, prognosis, drug efficacy evaluation, and patient outcomes. Similar to the application of AI in other mental disease, China, the United States and South Korea (32/52, 61.54%) were the countries with largest number of studies related to the use of AI in ADHD.
The data available in the application of AI in ADHD could be roughly divided into the following seven categories: demographic characteristics (gender, age, race, ethnicity, parental education, etc.); parent/teacher report questionnaire; neurocognitive characteristics; brain imaging (fMRI, sMRI, DTI) [20]; genetic data; EEG; eye tracking. Among them, 20 studies included MRI. MRI has demonstrated the possible physiological basis of the disease and is a potential predictor. ML or DL techniques may help identify reliable features and use this as a classification or diagnostic predictor [23]. Zhou, Lin [24] constructed a multimodal ML framework combining Boruta-based feature selection and multi-core learning, integrating sMRI, fMRI and DTI data for early diagnosis of ADHD. Then they used SVM to distinguish ADHD from healthy children. AUC of the model for diagnosing ADHD was 69.8%, and the classification ACC was 64.3%. The reported ACC of existing ADHD classification models varied, with most ranging between 60 and 90% [75]. Despite the success of MRI-based ML models, it has been found that models that incorporated demographic characteristics and/or parent/teacher questionnaires reported higher ACC in classification or diagnosis. One study evaluated parent/teacher ratings of executive function (from BRIEF’s Emergent Metacognition Composite score), behavioral/cognitive measures of executive function, measurements of cortical thickness in frontal subregions, and thickness and volume in the parietal cortex, two demographic characteristics (age and child sex), as well as a complete model with four categories. The results showed that the complete model with all the target features achieved a performance ACC of 0.994 in predicting ADHD diagnosis, with 0.926 derived from parent/teacher reports, which was considered critical in classifying ADHD [76]. ADHD was highly heritable (76% heritability) [77]. There was a study that combined multimodal MRI with candidate genetic data [25], including cortical morphology, diffusivity scalars, resting-state functional connectivity and polygenic risk score from norepinephrine, dopamine and glutamate genes. The integration of candidate single nucleotide polymorphism (SNP) data into the best model did not show a meaningful improvement in ACC. Existing studies of modeling using AI technique have all incorporated MRI diagnostic tools, in fact, it is important to acknowledge that neuroimaging data yields very little power [78]. There is still a need to focus on readily available behavioral/clinical data, including demographic information, subjective symptom ratings, and objective neuropsychological data. Integrated modeling approaches could facilitate the development of new approaches to ADHD classification and treatment. New types of data, such as eye tracking, could also be considered in the future in combination with clinical features.
Traditional ML and DL are two branches of AI. In this review, we investigated the characteristics of AI techniques present in the research. Most studies used ML, and the most commonly used algorithm was SVM (34, 65%), followed by RF (17, 33%). SVM by identifying the optimal hyperplane or by mapping nonlinear data into high-dimensional space using kernel functions to realize classification [79]. Its strength resides in its proficiency in managing small sample sizes, high-dimensional data, and nonlinear datasets efficiently, as exemplified when utilizing EEG to analyze ADHD [26]. Nevertheless, it is hindered by significant computational complexity and a heightened sensitivity to parameter adjustments. Conversely, Based on the voting mechanism of the integrated decision tree (DT), RF is good at processing large-scale multimodal data (such as when applying multi-center imaging and clinical data fusion to characterize ADHD) [80], does not require feature selection and is robust, and RF is known for its ability to perform well in classification and regression tasks [81]. However, the high complexity of the model leads to weak interpretability, and overfitting may occur in extreme cases [82]. The application scenarios of the two in the field of ADHD are significantly different: SVM is suitable for accurate classification tasks with limited data but complex features, while RF is more suitable for mining potential patterns in large-scale data. The sample size of ADHD research data is limited, so SVM is more suitable. DT and logistic regression (LR) are rarely used because they are difficult to cope with the high dimensional, non-linear and heterogeneous characteristics of ADHD data [27].
In contrast, DL was used 12 times (23.1%). K-fold CV was used in 34 (34/52, 65%) studies for AI model testing. In the early days, ML was widely used for its simplicity and high efficiency, owing to its advantages over traditional analytical methods based on mass-univariate statistics, especially considering the inter-correction among regions [16]. DL is a particular subtype of ML which is based on deep neural networks (DNNs). In contrast to ML technology, which requires manual extraction of features during image segmentation, DL employs artificial neural networks (ANNS) that allow direct processing of raw data and are particularly useful in identifying complex patterns in high-dimensional fMRI data to maximize model performance for related tasks [83]. Although there are few DL studies, their results are better than those of ML. There are several issues to be noted, one is the limitation of data volume, due to cross-sample reliability/validity and sensitivity and specificity limitations, ADHD diagnosis is primarily based on parent/teacher reports, neuroimaging is not yet part of the routine diagnosis process of ADHD [84]. Most of the MRI data in the published studies come from public databases, such as ADHD-200, the Study of Cognitive Development in the Adolescent Brain (ABCD), and Autism Brain Imaging Data Exchange (ABIDE), which have limited sample size and limited reproducibility [6, 85], the amount of data that is available is still not enough to meet the needs of DL. Secondly, it is the lack of transparency in the learning and testing process of DL that has led them to be called black boxes, and the interpretability of medical algorithms may have become a prerequisite for clinical adoption [86].
A large amount of the studies reported in this paper employed CV methods (44,84.6%), especially k-fold CV. CV, which is one input dataset split into parts, some of which are used as training classifiers (training data), and the remainder is used for validation (test data), this method is relatively economical, and could deal with overfitting and generalization problems to a certain extent [87]. However, due to the unbalanced nature of the number of features and the number of subjects in each study, as well as the high heterogeneity of the study sample, the generalization is still limited. Moreover, internal verification cannot guarantee the quality of ML model, it has no extrapolation [87]. Leave-one-out CV (LOOCV) is a special form of k-fold CV, which divides the data set into N subsets (N is the total number of samples). Only 1 sample is retained as the test set each time, and the remaining N-1 samples are repeated for N times. Finally, the average value of all test results is taken as the model evaluation index [28, 88]. This verification method can maximize the data utilization rate and is suitable for capturing the heterogeneity among ADHD individuals (such as the differences in neural markers of different subtypes). However, due to the high computational cost, it is not friendly to multi-modal high-dimensional data (such as fMRI), and can only be used for small data sets [28, 89]. AI requires large datasets to train models in order to avoid over-fitting and improve generalization. Only seven studies used datasets with more than 1,000 data points, and 21 studies used open datasets. In order to reflect the actual performance of the AI model in neuropsychiatric diseases, the model needed to be tested on multiple data sets to ensure its extrapolation [6]. AI models in future should be trained and validated in larger datasets [90]. DL has no advantage over ML in terms of classification and consumes more resources. However, the emergence of DL will further continue in the era of pediatric clinical studies because of its lesser reliance upon the existence of engineered features [91].
Comparison with previous studies
So far, we had retrieved five reviews on the use of AI in ADHD. A summative review explored the complex interaction of multiple cognitive, genetic and biological factors related to ADHD underling the ML-based algorithm [5]. The authors reported the significance of ML models in ADHD research. Loh, Ooi [92] conducted a systematic review by following PRISMA guidelines and focused on the diagnostic value of AI-based, they identified existing diagnostic tools for ADHD that are commonly used: EEG, MRI, questionnaires, exercise data, performance tests, etc. From the perspective of each diagnostic tool, the most commonly used features were discussed. Pereira-Sanchez and Castellanos [93] provided a brief narrative review of recent AI studies using sMRI and fMRI in ADHD patients, focusing on meta-analyses, large analyses, and proposed novel multimodal approaches. Periyasamy, Vibashan [20] provided a literature review on the application of AI in ADHD. In studies focusing on the use of MRI data, the feature extraction, dimensionality reduction/feature selection, and classification techniques were compared. Taspinar and Ozkurt [19] reported a review focusing on the inclusion of studies using sMRI data. Our scoping review focused on the role of AI techniques in the diagnosis, classification, and prediction of ADHD, following PRISMA guidelines. Provide the purpose and characteristics of all AI technologies listed in the study by reviewing the data sources and platforms used by the AI model. Hopefully, our findings will contribute to further ADHD research.
Limitations
This study had the following limitations. This review did not include articles related to the prognosis, treatment, and drug discovery of ADHD. The review was limited to journal articles written in English, while papers, review articles, conference abstracts, and review reports were excluded to reduce the complexity of the results. In fact, many research articles in the field of computers are published in full through conferences. In addition to popular public databases, half of the studies used private datasets, there was heterogeneity between studies in the methods and datasets used to generate assessment measures, such as the number of participants, data collection methods, and validation methods used. Finally, we only searched four commonly used databases, and there may have been omissions in some unsearched databases.
Conclusion
This scoping review is undertaken to support the existing evidence on the role of AI techniques in ADHD. We summarized AI models and algorithms for prediction, early diagnosis, and classification. Research into the application of AI to ADHD is still in its infancy, but early attempts to study ADHD using AI have shown promising results. Translating research into clinical practice still has a long way to go, and more explainable AI research and public education initiatives are needed. We believe that this review will help the scientific community better understand the application of AI techniques in ADHD.
Statements
Author contributions
Conceptualization, FC; methodology, BS; software, BS; validation, HH and BL; formal analysis, BS and FC; investigation, BW; resources, BW; data curation, HH and BL; writing – original draft preparation, BS and FC; writing – review and editing, BS, FC, HH, BL, and BW; visualization, FC; supervision, BW; project administration, BS. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.ebm-journal.org/articles/10.3389/ebm.2025.10238/full#supplementary-material
References
1.
RiglinLLeppertBLangleyKThaparAKO'DonovanMCDavey SmithGet alInvestigating attention-deficit hyperactivity disorder and autism spectrum disorder traits in the general population: what happens in adult life?J Child Psychol Psychiatry (2021) 62:449–57. 10.1111/jcpp.13297
2.
PolanczykGVSalumGASugayaLSCayeARohdeLA. Annual research review: a meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. J Child Psychol Psychiatry (2015) 56:345–65. 10.1111/jcpp.12381
3.
SibleyMHSwansonJMArnoldLEHechtmanLTOwensEBStehliAet alDefining ADHD symptom persistence in adulthood: optimizing sensitivity and specificity. J Child Psychol Psychiatry (2017) 58:655–62. 10.1111/jcpp.12620
4.
MahoneEMDencklaMB. Attention-deficit/hyperactivity disorder: a historical neuropsychological perspective. J Int Neuropsychological Soc (2017) 23:916–29. 10.1017/s1355617717000807
5.
CaoMMartinELiX. Machine learning in attention-deficit/hyperactivity disorder: new approaches toward understanding the neural mechanisms. Transl Psychiatry (2023) 13:236. 10.1038/s41398-023-02536-w
6.
EslamiTAlmuqhimFRaikerJSSaeedF. Machine learning methods for diagnosing autism spectrum disorder and attention- deficit/hyperactivity disorder using functional and structural MRI: a survey. Front Neuroinform (2020) 14:575999. 10.3389/fninf.2020.575999
7.
KessiMDuanHXiongJChenBHeFYangLet alAttention-deficit/hyperactive disorder updates. Front Mol Neurosci (2022) 15:925049. 10.3389/fnmol.2022.925049
8.
LuoYWeibmanDHalperinJMLiX. A review of heterogeneity in attention deficit/hyperactivity disorder (ADHD). Front Hum Neurosci (2019) 13:42. 10.3389/fnhum.2019.00042
9.
ElamJSGlasserMFHarmsMPSotiropoulosSNAnderssonJLRBurgessGCet alThe human connectome project: a retrospective. NeuroImage (2021) 244:118543. 10.1016/j.neuroimage.2021.118543
10.
ConnaughtonMWhelanRO'HanlonEMcGrathJ. White matter microstructure in children and adolescents with ADHD. NeuroImage Clin (2022) 33:102957. 10.1016/j.nicl.2022.102957
11.
DemontisDWaltersRKMartinJMattheisenMAlsTDAgerboEet alDiscovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet (2019) 51:63–75. 10.1038/s41588-018-0269-7
12.
LiuLFengXLiHCheng LiSQianQWangY. Deep learning model reveals potential risk genes for ADHD, especially Ephrin receptor gene EPHA5. Brief Bioinform (2021) 22:bbab207. 10.1093/bib/bbab207
13.
KohJEWOoiCPLim-AshworthNSVicneshJTorHTLihOSet alAutomated classification of attention deficit hyperactivity disorder and conduct disorder using entropy features with ECG signals. Comput Biol Med (2022) 140:105120. 10.1016/j.compbiomed.2021.105120
14.
YooJHKangCLimJSWangBChoiCHHwangHet alDevelopment of an innovative approach using portable eye tracking to assist ADHD screening: a machine learning study. Front Psychiatry (2024) 15:1337595. 10.3389/fpsyt.2024.1337595
15.
KimWPKimHJPackSPLimJHChoCHLeeHJ. Machine learning-based prediction of attention-deficit/hyperactivity disorder and sleep problems with wearable data in children. JAMA Netw Open (2023) 6:e233502. 10.1001/jamanetworkopen.2023.3502
16.
VieiraSPinayaWHMechelliA. Using deep learning to investigate the neuroimaging correlates of psychiatric and neurological disorders: methods and applications. Neurosci and Biobehavioral Rev (2017) 74:58–75. 10.1016/j.neubiorev.2017.01.002
17.
GaggioliA. Quality of experience in real and virtual environments: some suggestions for the development of positive technologies. Stud Health Technol Inform (2012) 181:177–81. 10.3233/978-1-61499-121-2-177
18.
ZhangZLiGXuYTangX. Application of artificial intelligence in the MRI classification task of human brain neurological and psychiatric diseases: a scoping review. Diagnostics (Basel, Switzerland) (2021) 11:1402. 10.3390/diagnostics11081402
19.
TaspinarGOzkurtN. A review of ADHD detection studies with machine learning methods using rsfMRI data. NMR Biomed (2024) 37:e5138. 10.1002/nbm.5138
20.
PeriyasamyRVibashanVSVargheseGTAleemMA. Machine learning techniques for the diagnosis of attention-deficit/hyperactivity disorder from magnetic resonance imaging: a concise review. Neurol India (2021) 69:1518–23. 10.4103/0028-3886.333520
21.
TriccoACLillieEZarinWO'BrienKKColquhounHLevacDet alPRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med (2018) 169:467–73. 10.7326/m18-0850
22.
CumpstonMLiTPageMJChandlerJWelchVAHigginsJPet alUpdated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev (2019) 10:Ed000142. 10.1002/14651858.ED000142
23.
ÖztekinIGaricDBayatMHernandezMLFinlaysonMAGrazianoPAet alStructural and diffusion-weighted brain imaging predictors of attention-deficit/hyperactivity disorder and its symptomology in very young (4- to 7-year-old) children. Eur J Neurosci (2022) 56:6239–57. 10.1111/ejn.15842
24.
ZhouXLinQGuiYWangZLiuMLuH. Multimodal MR images-based diagnosis of early adolescent attention-deficit/hyperactivity disorder using multiple kernel learning. Front Neurosci (2021) 15:710133. 10.3389/fnins.2021.710133
25.
YooJHKimJIKimBNJeongB. Exploring characteristic features of attention-deficit/hyperactivity disorder: findings from multi-modal MRI and candidate genetic data. Brain Imaging Behav (2020) 14:2132–47. 10.1007/s11682-019-00164-x
26.
KimJWKimBNKimJIYangCMKwonJ. Electroencephalogram (EEG) based prediction of attention deficit hyperactivity disorder (ADHD) using machine learning. Neuropsychiatr Dis Treat (2025) 21:271–9. 10.2147/ndt.s509094
27.
Garcia-ArgibayMZhang-JamesYCorteseSLichtensteinPLarssonHFaraoneSV. Predicting childhood and adolescent attention-deficit/hyperactivity disorder onset: a nationwide deep learning approach. Mol Psychiatry (2023) 28:1232–9. 10.1038/s41380-022-01918-8
28.
WangXHJiaoYLiL. Identifying individuals with attention deficit hyperactivity disorder based on temporal variability of dynamic functional connectivity. Sci Rep (2018) 8:11789. 10.1038/s41598-018-30308-w
29.
TanLGuoXRenSEpsteinJNLuLJ. A computational model for the automatic diagnosis of attention deficit hyperactivity disorder based on functional brain volume. Front Comput Neurosci (2017) 11:75. 10.3389/fncom.2017.00075
30.
QureshiMNMinBJoHJLeeB. Multiclass classification for the differential diagnosis on the ADHD subtypes using recursive feature elimination and hierarchical extreme learning machine: structural MRI study. PLoS One (2016) 11:e0160697. 10.1371/journal.pone.0160697
31.
GhiassianSGreinerRJinPBrownMR. Using functional or structural magnetic resonance images and personal characteristic data to identify ADHD and autism. PLoS One (2016) 11:e0166934. 10.1371/journal.pone.0166934
32.
DudaMMaRHaberNWallDP. Use of machine learning for behavioral distinction of autism and ADHD. Transl Psychiatry (2016) 6:e732. 10.1038/tp.2015.221
33.
RezaeiMZareHHakimdavoodiHNasseriSHebraniP. Classification of drug-naive children with attention-deficit/hyperactivity disorder from typical development controls using resting-state fMRI and graph theoretical approach. Front Hum Neurosci (2022) 16:948706. 10.3389/fnhum.2022.948706
34.
MuthuramanMMoliadzeVBoecherLSiemannJFreitagCMGroppaSet alMultimodal alterations of directed connectivity profiles in patients with attention-deficit/hyperactivity disorders. Sci Rep (2019) 9:20028. 10.1038/s41598-019-56398-8
35.
UyulanCErguzelTTTurkOFarhadSMetinBTarhanN. A class activation map-based interpretable transfer learning model for automated detection of ADHD from fMRI data. Clin EEG Neurosci (2023) 54:151–9. 10.1177/15500594221122699
36.
HellerMDRootsKSrivastavaSSchumannJSrivastavaJHaleTS. A machine learning-based analysis of game data for attention deficit hyperactivity disorder assessment. Games Health J (2013) 2:291–8. 10.1089/g4h.2013.0058
37.
SlobodinOYahavIBergerI. A machine-based prediction model of ADHD using CPT data. Front Hum Neurosci (2020) 14:560021. 10.3389/fnhum.2020.560021
38.
DasWKhannaS. A robust machine learning based framework for the automated detection of ADHD using pupillometric biomarkers and time series analysis. Sci Rep (2021) 11:16370. 10.1038/s41598-021-95673-5
39.
LuoNLuoXZhengSYaoDZhaoMCuiYet alAberrant brain dynamics and spectral power in children with ADHD and its subtypes. Eur Child Adolesc Psychiatry (2023) 32:2223–34. 10.1007/s00787-022-02068-6
40.
FaeddaGLOhashiKHernandezMMcGreeneryCEGrantMCBaroniAet alActigraph measures discriminate pediatric bipolar disorder from attention-deficit/hyperactivity disorder and typically developing controls. J Child Psychol Psychiatry (2016) 57:706–16. 10.1111/jcpp.12520
41.
ColbyJBRudieJDBrownJADouglasPKCohenMSShehzadZ. Insights into multimodal imaging classification of ADHD. Front Syst Neurosci (2012) 6:59. 10.3389/fnsys.2012.00059
42.
LohaniDCRanaB. ADHD diagnosis using structural brain MRI and personal characteristic data with machine learning framework. Psychiatry Res Neuroimaging (2023) 334:111689. 10.1016/j.pscychresns.2023.111689
43.
BrownMRSidhuGSGreinerRAsgarianNBastaniMSilverstonePHet alADHD-200 Global Competition: diagnosing ADHD using personal characteristic data can outperform resting state fMRI measurements. Front Syst Neurosci (2012) 6:69. 10.3389/fnsys.2012.00069
44.
YasumuraAOmoriMFukudaATakahashiJYasumuraYNakagawaEet alApplied machine learning method to predict children with ADHD using prefrontal cortex activity: a multicenter study in Japan. J Atten Disord (2020) 24:2012–20. 10.1177/1087054717740632
45.
Ter-MinassianLVianiNWickershamACrossLStewartRVelupillaiSet alAssessing machine learning for fair prediction of ADHD in school pupils using a retrospective cohort study of linked education and healthcare data. BMJ Open (2022) 12:e058058. 10.1136/bmjopen-2021-058058
46.
DaiDWangJHuaJHeH. Classification of ADHD children through multimodal magnetic resonance imaging. Front Syst Neurosci (2012) 6:63. 10.3389/fnsys.2012.00063
47.
YangCMShinJKimJILimYBParkSHKimBN. Classifying children with ADHD based on prefrontal functional near-infrared spectroscopy using machine learning. Clin Psychopharmacol Neurosci (2023) 21:693–700. 10.9758/cpn.22.1025
48.
Varela CasalPLorena EspositoFMorata MartínezICapdevilaASolé PuigMde la OsaNet alClinical validation of eye vergence as an objective marker for diagnosis of ADHD in children. J Atten Disord (2019) 23:599–614. 10.1177/1087054717749931
49.
AbibullaevBAnJ. Decision support algorithm for diagnosis of ADHD using electroencephalograms. J Med Syst (2012) 36:2675–88. 10.1007/s10916-011-9742-x
50.
LeeWLeeSLeeDJunKAhnDHKimMS. Deep learning-based ADHD and ADHD-RISK classification technology through the recognition of children's abnormal behaviors during the robot-led ADHD screening game. Sensors (Basel) (2022) 23:278. 10.3390/s23010278
51.
EsasMYLatifoğluF. Detection of ADHD from EEG signals using new hybrid decomposition and deep learning techniques. J Neural Eng (2023) 20:036028. 10.1088/1741-2552/acc902
52.
BledsoeJCXiaoCChaovalitwongseAMehtaSGrabowskiTJSemrud-ClikemanMet alDiagnostic classification of ADHD versus control: support vector machine classification using brief neuropsychological assessment. J Atten Disord (2020) 24:1547–56. 10.1177/1087054716649666
53.
HaqueUMKabirEKhanamR. Early detection of paediatric and adolescent obsessive-compulsive, separation anxiety and attention deficit hyperactivity disorder using machine learning algorithms. Health Inf Sci Syst (2023) 11:31. 10.1007/s13755-023-00232-z
54.
PengXLinPZhangTWangJ. Extreme learning machine-based classification of ADHD using brain structural MRI data. PLoS One (2013) 8:e79476. 10.1371/journal.pone.0079476
55.
QureshiMNIOhJMinBJoHJLeeB. Multi-modal, multi-measure, and multi-class discrimination of ADHD with hierarchical feature extraction and extreme learning machine using structural and functional brain MRI. Front Hum Neurosci (2017) 11:157. 10.3389/fnhum.2017.00157
56.
O’MahonyNFlorentino-LianoBCarballoJJBaca-GarcíaERodríguezAA. Objective diagnosis of ADHD using IMUs. Med Eng and Phys (2014) 36:922–6. 10.1016/j.medengphy.2014.02.023
57.
LinHHaiderSPKaltenhauserSMozayanAMalhotraAConstableRTet alPopulation level multimodal neuroimaging correlates of attention-deficit hyperactivity disorder among children. Front Neurosci (2023) 17:1138670. 10.3389/fnins.2023.1138670
58.
KimJWSharmaVRyanND. Predicting methylphenidate response in ADHD using machine learning approaches. Int J Neuropsychopharmacol (2015) 18:pyv052. 10.1093/ijnp/pyv052
59.
ChenHSongYLiX. Use of deep learning to detect personalized spatial-frequency abnormalities in EEGs of children with ADHD. J Neural Eng (2019) 16:066046. 10.1088/1741-2552/ab3a0a
60.
MikolasPVahidABernardoniFSüßMMartiniJBesteCet alTraining a machine learning classifier to identify ADHD based on real-world clinical data from medical records. Sci Rep (2022) 12:12934. 10.1038/s41598-022-17126-x
61.
ChengYHanLWuLChenJSunHWenGet alEffect of first-line serplulimab vs placebo added to chemotherapy on survival in patients with extensive-stage small cell lung cancer: the ASTRUM-005 randomized clinical trial. Jama (2022) 328:1223–32. 10.1001/jama.2022.16464
62.
CrippaASalvatoreCMolteniEMauriMSalandiATrabattoniSet alThe utility of a computerized algorithm based on a multi-domain profile of measures for the diagnosis of attention deficit/hyperactivity disorder. Front Psychiatry (2017) 8:189. 10.3389/fpsyt.2017.00189
63.
Navarro-SoriaIRico-JuanJRJuárez-Ruiz de MierRLavigne-CervanR. Prediction of attention deficit hyperactivity disorder based on explainable artificial intelligence. Appl Neuropsychol Child (2024) 1–14. 10.1080/21622965.2024.2336019
64.
LiuZLiJZhangYWuDHuoYYangJet alAuxiliary diagnosis of children with attention-deficit/hyperactivity disorder using eye-tracking and digital biomarkers: case-control study. JMIR Mhealth Uhealth (2024) 12:e58927. 10.2196/58927
65.
ChiangHLWuCSChenCLTsengWYIGauSS. Machine-learning-based feature selection to identify attention-deficit hyperactivity disorder using whole-brain white matter microstructure: a longitudinal study. Asian J Psychiatry (2024) 97:104087. 10.1016/j.ajp.2024.104087
66.
ZhangKFYehSCHsiao-Kuang WuEXuXTsaiHJChenCC. Fusion of multi-task neurophysiological data to enhance the detection of attention- deficit/hyperactivity disorder. IEEE J Transl Eng Health Med (2024) 12:668–74. 10.1109/jtehm.2024.3435553
67.
BlairRJRBashford-LargoJDominguezADobbertinMBlairKSBajajS. Using machine learning to determine a functional classifier of reward responsiveness and its association with adolescent psychiatric symptomatology. Psychol Med (2024) 54:4212–21. 10.1017/s003329172400240x
68.
WangGLiWHuangSChenZ. A prospective study of an early prediction model of attention deficit hyperactivity disorder based on artificial intelligence. J Atten Disord (2024) 28:302–9. 10.1177/10870547231211360
69.
ChiuYHLeeYHWangSYOuyangCSWuRCYangRCet alObjective approach to diagnosing attention deficit hyperactivity disorder by using pixel subtraction and machine learning classification of outpatient consultation videos. J Neurodevelopmental Disord (2024) 16:71. 10.1186/s11689-024-09588-z
70.
OuyangCSYangRCWuRCChiangCTChiuYHLinLC. Objective and automatic assessment approach for diagnosing attention-deficit/hyperactivity disorder based on skeleton detection and classification analysis in outpatient videos. Child Adolesc Psychiatry Ment Health (2024) 18:60. 10.1186/s13034-024-00749-5
71.
ChenICChangCLChangMHKoLW. The utility of wearable electroencephalography combined with behavioral measures to establish a practical multi-domain model for facilitating the diagnosis of young children with attention-deficit/hyperactivity disorder. J Neurodevelopmental Disord (2024) 16:62. 10.1186/s11689-024-09578-1
72.
PerezDLBiffiACamprodonJACaplanDNChemaliZKritzerMDet alTelemedicine in behavioral neurology-neuropsychiatry: opportunities and challenges catalyzed by COVID-19. Cogn Behav Neurol (2020) 33:226–9. 10.1097/wnn.0000000000000239
73.
GrazioliSCrippaARosiECandelieriACeccarelliSBMauriMet alExploring telediagnostic procedures in child neuropsychiatry: addressing ADHD diagnosis and autism symptoms through supervised machine learning. Eur Child Adolesc Psychiatry (2024) 33:139–49. 10.1007/s00787-023-02145-4
74.
AllesøeRLThompsonWKBybjerg-GrauholmJHougaardDMNordentoftMWergeTet alDeep learning for cross-diagnostic prediction of mental disorder diagnosis and prognosis using Danish nationwide register and genetic data. JAMA psychiatry (2023) 80:146–55. 10.1001/jamapsychiatry.2022.4076
75.
QuaakMvan de MortelLThomasRMvan WingenG. Deep learning applications for the classification of psychiatric disorders using neuroimaging data: systematic review and meta-analysis. NeuroImage Clin (2021) 30:102584. 10.1016/j.nicl.2021.102584
76.
ÖztekinIFinlaysonMAGrazianoPADickAS. Is there any incremental benefit to conducting neuroimaging and neurocognitive assessments in the diagnosis of ADHD in young children? A machine learning investigation. Developmental Cogn Neurosci (2021) 49:100966. 10.1016/j.dcn.2021.100966
77.
FaraoneSVLarssonH. Genetics of attention deficit hyperactivity disorder. Mol Psychiatry (2019) 24:562–75. 10.1038/s41380-018-0070-0
78.
HoogmanMBraltenJHibarDPMennesMZwiersMPSchwerenLSJet alSubcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: a cross-sectional mega-analysis. The lancet Psychiatry (2017) 4:310–9. 10.1016/s2215-0366(17)30049-4
79.
BarberisEKhosoSSicaAFalascaMGennariADonderoFet alPrecision medicine approaches with metabolomics and artificial intelligence. Int J Mol Sci (2022) 23:11269. 10.3390/ijms231911269
80.
SharmaCMChariarVM. Diagnosis of mental disorders using machine learning: literature review and bibliometric mapping from 2012 to 2023. Heliyon (2024) 10:e32548. 10.1016/j.heliyon.2024.e32548
81.
HuJSzymczakS. A review on longitudinal data analysis with random forest. Brief Bioinform (2023) 24:bbad002. 10.1093/bib/bbad002
82.
Roman-NaranjoPParra-PerezAMLopez-EscamezJA. A systematic review on machine learning approaches in the diagnosis and prognosis of rare genetic diseases. J Biomed Inform (2023) 143:104429. 10.1016/j.jbi.2023.104429
83.
EitelFSchulzMASeilerMWalterHRitterK. Promises and pitfalls of deep neural networks in neuroimaging-based psychiatric research. Exp Neurol (2021) 339:113608. 10.1016/j.expneurol.2021.113608
84.
TakahashiNIshizukaKInadaT. Peripheral biomarkers of attention-deficit hyperactivity disorder: current status and future perspective. J Psychiatr Res (2021) 137:465–70. 10.1016/j.jpsychires.2021.03.012
85.
ButtonKSIoannidisJPMokryszCNosekBAFlintJRobinsonESet alPower failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci (2013) 14:365–76. 10.1038/nrn3475
86.
LapuschkinSWäldchenSBinderAMontavonGSamekWMüllerKR. Unmasking Clever Hans predictors and assessing what machines really learn. Nat Commun (2019) 10:1096. 10.1038/s41467-019-08987-4
87.
HoSYPhuaKWongLBin GohWW. Extensions of the external validation for checking learned model interpretability and generalizability. Patterns (New York, NY) (2020) 1:100129. 10.1016/j.patter.2020.100129
88.
ShaoZErMJWangN. An efficient leave-one-out cross-validation-based extreme learning machine (ELOO-ELM) with minimal user intervention. IEEE Trans Cybern (2016) 46:1939–51. 10.1109/tcyb.2015.2458177
89.
WangXHJiaoYLiL. Diagnostic model for attention-deficit hyperactivity disorder based on interregional morphological connectivity. Neurosci Lett (2018) 685:30–4. 10.1016/j.neulet.2018.07.029
90.
Zhang-JamesYHelminenECLiuJBusattoGFCalvoACercignaniMet alEvidence for similar structural brain anomalies in youth and adult attention-deficit/hyperactivity disorder: a machine learning analysis. Transl Psychiatry (2021) 11:82. 10.1038/s41398-021-01201-4
91.
SargolzaeiS. Can deep learning hit a moving target? A scoping review of its role to study neurological disorders in children. Front Comput Neurosci (2021) 15:670489. 10.3389/fncom.2021.670489
92.
LohHWOoiCPBaruaPDPalmerEEMolinariFAcharyaUR. Automated detection of ADHD: current trends and future perspective. Comput Biol Med (2022) 146:105525. 10.1016/j.compbiomed.2022.105525
93.
Pereira-SanchezVCastellanosFX. Neuroimaging in attention-deficit/hyperactivity disorder. Curr Opin Psychiatry (2021) 34:105–11. 10.1097/yco.0000000000000669
Summary
Keywords
artificial intelligence, attention deficit/hyperactivity disorder, machine learning, deep learning, review method
Citation
Sun B, Cai F, Huang H, Li B and Wei B (2025) Artificial intelligence for children with attention deficit/hyperactivity disorder: a scoping review. Exp. Biol. Med. 250:10238. doi: 10.3389/ebm.2025.10238
Received
12 May 2024
Accepted
28 March 2025
Published
24 April 2025
Volume
250 - 2025
Updates
Copyright
© 2025 Sun, Cai, Huang, Li and Wei.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bing Wei, weibing7112@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.